Summary
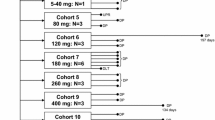
We have conducted a clinical and pharmacokinetic trial of the novel podophyllotoxin derivative NK611 administered orally for 21 consecutive days. The treatment was repeated every 35 days. Eighteen patients were included into the study, all of whom were eligible. Due to early progression of tumor disease in two patients, 16 patients were évaluable for toxicity [7 female, 9 male, median age 64 years (range: 44 to 73)]. Dose escalation steps were 5 mg/day [105 mg per cycle (pc)], 10 mg/day (210 mg pc), 12.5 mg/day (265 mg pc) and 15 mg/day (315 mg pc). A total of 37 courses was administered. Toxicity was evaluated using NCI-CTC criteria. Granulocytopenia was the main hematologic toxicity. Other hematologic toxicities were sporadic. Non-hematologic toxicities were mild and consisted of grade 1 nausea and grade 2 alopecia. Pharmacokinetic analyses were performed in six patients each treated with 10 mg/day and 12.5 mg per day, and in one patient treated with 15 mg/day. Using a two-compartment model, t1/2α ranged from 0.47 to 1.54 h and t1/2β from 2.0–11.6 h. Mean values for C max and AUC were 1.47 ± 0.331 μg/ml and 13.67±3.81 μg/ml·h. No objective tumor responses were observed. However, one patient with metastatic breast cancer had stable disease for twelve months. We conclude that the Maximum Tolerated Dose of NK611 administered daily for 21 consecutive days is 12.5 mg/day. The Dose-Limiting Toxicity is granulocytopenia. The recommended dose for further clinical Phase II studies is 10 mg/day.
Similar content being viewed by others
References
Machida M, Tanaka S, Nakamori K: Simultaneous determination of NK611, a novel water-soluble derivative of etoposide, and its metabolite (DeNK611) in dogs plasma by column-switching high performance chromatography. Biomedic Chromatograph 7:82–85, 1993
Asta Medica GmbH. Investigator's drug brochure: NK611. Asta Medica GmbH 1993, p. 35
Ekimoto H, Okamoto K, Kusuma K, Mashiba H, Takeuchi T: Treatment schedule dependency of antitumor effects of NK611, a novel water-soluble analog of etoposide, on human xenografts. Proc Amer Assoc Cancer Res 33:436, 1992
Pagani O, Zucchetti M, Sessa C, De Jong J, D'Incalci M, De Fusco M, Kaeser-Fröhlich A, Hanauske A-R, Cavalli F: Phase I clinical and pharmacokinetic study of oral NK611, a new podophyllotoxin derivative. In press 1996
Hainsworth JD, Greco FA: Etoposide: twenty years later. Ann Oncol 6:325–341, 1995
Ekimoto H, Okamoto K, Maruyma S, Mashiba S, Nakamori K, Takeuchi T: Preclinical characterization of NK611, a novel water-soluble etoposide analog. Ann Oncol 3(suppl. 1):98, 1992
Hanauske A-R, Wüster KC, Lehmer A, Rotter M, Schneider P, Kaeser-Fröhlich A, Rastetter J, Depenbrock H: Activity of NK611, a new epipodophyllotoxin derivative, against colony forming units from freshly explanted human tumors in vitro. Eur J Cancer 31A:1677–1681, 1995
Zucchetti M, De Fusco M, Sessa C, Fröhlich A, Reichert S, D'Iticalci M: High-performance liquid chromatographic assay for determination of the novel podophyllotoxin derivative dimethylaminoetoposide (NK611) in human plasma. J Chromatogr 654B:97–102, 1994
De Fusco M, D'Incalci M, Gentili B, Reichert S, Zucchetti M: High performance liquid chromatographic assay for the determination of the novel etoposide derivative dimethylaminoetoposide (NK611) and its metabolites in urine in cancer patients. J Chromatogr B 664:409–412, 1995
Sacchi-Landriani G, Guardabasso V, Rocchetti M: NI-Fit: a microcomputer program for non-linear fitting. Comput Programs Biomed 16:35–38, 1983
Greco FA: Chronic etoposide administration: overview of clinical experience. Canc Treat Rev 19(Suppl C):35–45, 1993
Schilling T, Mross K, Berdel WE, Manegold C, Fiebig HH, Rastetter J, Hanauske A-R: Phase I clinical and pharmacokinetic trial of the podophyllotoxin derivative NK611. Ann Oncol 5(Suppl 5):193, 1994
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raßmann, I., Schrödel, H., Schilling, T. et al. Clinical and pharmacokinetic phase I trial of oral dimethylaminoetoposide (NK611) administered for 21 days every 35 days. Invest New Drugs 14, 379–386 (1996). https://doi.org/10.1007/BF00180814
Issue Date:
DOI: https://doi.org/10.1007/BF00180814