Summary
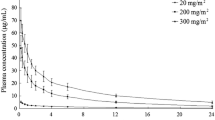
An HPLC analytical method was applied to the determination of plasma concentrations of 5,6-dihydro-5-azacytidine (NSC 264880, DHAC) in two foxhounds after a rapid intravenous infusion of 300 mg/kg DHAC. The dose employed is the mouse equivalent LD10 dose which results in mild reversible toxicity in the dog. The decline in DHAC plasma concentrations was greater than three log decades after dosing. The plasma concentration-time data was computer-fitted by NONLIN to a three compartment open model with first-order elimination using the equations for a short intravenous infusion. The half-lives corresponding to the three exponential phases were: t1/2α =5.78 min, t1/2β =1.57 h and t1/2γ =22.0 h in dog 1 and t1/2α =7.41 min, t1/2β =2.25 h and t1/2γ =21.6 h in dog 2. The terminal phase of the plasma concentration time profiles represented a minor contribution (2.2–3.2%) to the total area under the curve. The plasma concentration time data for the first 12 h after dosing was computer fitted to a two compartment open model. The initial and terminal half-lives determined from the two compartment fits were similar to the t1/2α and t1/2β values of the fits to the three compartment open model. Similar total body clearance values were calculated from the areas under the plasma concentration time curves from time zero to infinity for the computer fits to the three and two compartment models, respectively. Thus, for practical purposes, it appears feasible to define adequately the total body clearance of DHAC by analysis of the plasma concentration time data during the time interval in which the plasma concentration is described as a bi-exponential equation. Renal excretion of the parent drug is the principal route of excretion of DHAC from the dog.
Similar content being viewed by others
References
Beisler JA, Abbasi MM, Driscoll JS: Dihydro-5-azacytidine hydrochloride, a biologically active and chemically stable analog of 5-azacytidine. Cancer Treat Rep 60:1671–1674, 1976
Beisler JA, Abbasi MM, Kelley JA, Driscoll JS: Synthesis and antitumor activity of dihydro-5-azacytidine, a hydrolytically stable analogue of 5-azacytidine. J Med Chem 20:806–812, 1977
Beisler JA, Abbasi MM, Kelley JA, Driscoll JS: Chemistry of antitumor triazine nucleosides. An improved synthesis of dihydro-5-azacytidine. J Carbohydrates Nucleosides Nucleotides 4:281–199, 1977
Israili ZH, Vogler WR, Mingioli ES, Pirkle JL, Smithwick RW, Goldstein JH: The disposition and pharmacokinetics in humans of 5-azacytidine administered intravenously as a bolus or by continuous infusion. Cancer Res 36:1453–1461, 1976
Chan KK, Giannini DD, Staroscik JA, Sadee W: 5-Azacytidine hydrolysis kinetics measured by high-pressure liquid chromatography and 13C-NMR spectroscopy. J Pharm Sci 68:807–812, 1979
Pithova P, Piskala A, Pitha J, Sorm F: Nucleic acid components and their analogues. LXVI. Hydrolysis of 5-azacytidine and its connection with biological activity. Collection Czechoslov Chem Communs 30:2801–2811, 1965
Von Hoff DD, Slavik M: 5-Azacytidine — a new anticancer drug with significant activity in acute myeloblastic leukemia. In S Garattini, A Golden, F Hawking and IJ Kopin (eds): Advances in Pharmacology and Chemotherapy Vol 14. Academic Press, New York, 1977, pp 317–322
Investigational Drug Branch: Clinical Brochure, Dihydro-5-azacytidine Hydrochloride (DHAC), NSC 264880. National Cancer Institute, Bethesda, 1981, pp 9–14.
Investigational Drug Branch: Clinical Brochure, Dihydro-5-azacytidine Hydrochloride (DHAC), NSC 264880. National Cancer Institute, Bethesda, 1981, p 16
Malspeis L, Cheng H, Bhat HB, Staubus AE: High-performance liquid Chromatographic analysis of 5,6-dihydro-5-azacytidine in biological fluids. J Chromatogr, submitted
Metzler CM, Elfring GK, McEwen AJ: A package of computer programs for pharmacokinetic modeling. Biometrics 30:562–563, 1974
Altman PL, Dittmer DS (eds): Biology Data Book, 2nd Ed, Vol III. Federation of American Society for Experimental Biology, Bethesda, 1974, p 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Malspeis, L., Cheng, H. & Staubus, A.E. Pharmacokinetics of 5,6-dihydro-5-azacytidine (NSC-264880) in the foxhound. Invest New Drugs 1, 47–58 (1983). https://doi.org/10.1007/BF00180191
Issue Date:
DOI: https://doi.org/10.1007/BF00180191