Summary
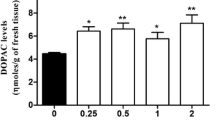
In this study, two ester pro-drugs of dopamine (DA) were synthesized and evaluated. These derivatives were the monobenzoyl (MBDA) and dibenzoyl (DBDA) esters of DA. MBDA was 300-fold and DBDA was 20,000-fold more lipophilic than DA itself. The half-lives of hydrolysis for MBDA and DBDA at physiologic pH and temperature were 15 and 420 min respectively. These compounds were radiolabelled and their uptake into brain measured. 14C-DBDA penetrated the brain rapidly; 0.28% of the dose injected was taken up per gram of brain tissue at 5 min. However DBDA did not produce measurable increases in DA levels in the brain. 14C-MBDA was found not to penetrate the brain. However, when MBDA was administered intracerebroventricularly (i.c.v.) to rats, it caused DOPAC levels to increase significantly both in the striatum and in the rest of the brain. The increase in the amount of DOPAC measured in the striatum was 3 to 10-fold greater than that seen in the rest of the brain. In rats that were pretreated with the MAO inhibitor, pargyline, MBDA given i.c.v. caused increases in DA levels in both the striatum and in the rest of the brain. The increased DA levels in striatum were considerably greater than those seen in the rest of the brain. From these results, it is inferred that MBDA is being hydrolyzed in vivo in the brain to form DA which is then taken up into dopaminergic neurons. Given this, it seems likely that an ester pro-drug of DA can be obtained that will have sufficient lipophilicity to penetrate the brain as well as a rate of hydrolysis that will produce increases in DA in the brain.
Similar content being viewed by others
References
Arnett CD, Fowler JS, Wolf AP, MacGregor RR (1983) Specific binding of 11C-spiroperidol in rat brain in vivo. J Neurochem 40:455–459
Bianchine JR (1980) Drugs for Parkinson's disease. In: Gilman AG, Goodman LS, Gilman A (eds) The Pharmacological Basis of Therapeutics. Macmillan, New York, pp 475–487
Borgman RJ, McPhillips JJ, Stitzel RE, Goodman IJ (1973) Synthesis and pharmacology of centrally acting dopamine derivatives and analogs in relation to Parkinson's disease. J Med Chem 16:630–633
Borgman RJ, Baldessarini RJ, Walton KG (1976) Diester derivatives as apomorphine pro-drugs. J Med Chem 19:717–719
Capon B, Ghosh BC (1966) The mechanism of the hydrolysis of phenyl salicylate and catechol monobenzoate in the presence and absence of borate ions. J Chem Soc (B) 473–478
Casagrande C, Ferrari G (1973) 3,4-O-Diacyl derivatives of dopamine. Il Farmaco 28:143–148
Casagrande C, Santangelo F, Saini C, Doggi F, Gerli F, Cerri O (1986) Synthesis and chemical properties of ibopamine and of related esters of N-substituted dopamines-synthesis of ibopamine metabolites. Arzneim-Forsch (Drug Res) 36:291–303
Conway PG, Tejani-Butt SM, Brunswick DJ (1987) Interaction of beta-adrenergic agonists and antagonists with brain beta-adrenergic receptors in vivo. J Pharmacol Exp Ther 241:755–762
Cumming P, Boyes BE, Martin WRW, Adam M, Grierson J, Ruth T, McGeer EG (1987a) The metabolism of 18F-6-fluoro-l-3,4-dihydroxyphenylalanine in the hooded rat. J Neurochem 48:601–608
Cumming P, Boyes BE, Martin WRW, Adam M, Ruth T, McGeer EG (1987b) Altered metabolism of 18F-6-fluorodopa in the hooded rat following inhibition of catechol-O-methyltransferase with U-0521. Biochem Pharmacol 36:2527–2531
Dax EM, Partilla JS (1982) Adrenergic ligand liposolubility in membranes: Direct assessment in a beta-adrenergic binding system. Mol Pharmacol 22:5–7
Dischino DD, Welch MJ, Kilbourn MR, Raichle ME (1983) Relationship between lipophilicity and brain extraction of C-11 labeled radiopharmaceuticals. J Nucl Med 24:1030–1038
Firnau G, Sood S, Chirakal R, Nahmias C, Garnett ES (1987) Cerebral metabolism of 6–18 F-fluoro-L-3,4-dihydroxyphenylalanine in the primate. J Neurochem 48:1077–1082
Fuller EJ (1963) Catalysis by catechol monoanion. J Amer Chem Soc 85:1777–1780
Gardner CR, Alexander J (1985) Prodrug approaches to drug targeting: Past accomplishments and future potential. In: Buri P, Gumman A (eds) Drug Targeting. Elsevier, Amsterdam, pp 145–164
Garnett ES, Firnau G, Chan PFH, Sood S, Belbeck LW (1987) 18 F-fluoro-dopa, an analogue of dopa, and its use in direct external measurements of storage, degradation and turnover of intracerebral dopamine. Proc Natl Acad Sci USA 75:464–467
Hansch C, Steward AR, Anderson SM, Bentley D (1967) The parabolic dependence of drug action upon lipophilic character as revealed by a study of hypnotics. J Med Chem 11:1–11
Hansch C, Bjorkroth JP, Leo A (1987) Hydrophobicity and central nervous system agents: On the principle of minimal hydrophobicity in drug action. J Pharm Sci 76:663–687
Hansen B (1963) Kinetics of the alkaline hydrolysis of catechol monoacetate and some derivatives. Acta Chem Scand 17: 1375–1379
Horn AS, deKaste D, Dijkstra D, Rollema H, Feenstra M, Westerink BHC, Grol C, Westerink A (1978) A new dopaminergic prodrug. Nature 276:405–407
Kristoffersson J, Svensson LA, Tegner K (1974) Drug latentiation of terbutaline. Acta Pharm Suec 11:427–438
Marrel C, Boss G, Testa B, van de Waterbeemd H, Cooper D, Jenner P, Marsden CD (1985) l-Dopa esters as potential prodrugs. Eur J Med Chem 20:467–470
Previero A, Barry LG, Coletti-Previero MA (1972) Specific O-acylation of hydroxylamino acids in presence of free amino groups. Biochem Biophys Acta 263:7–13
Shashoua VE, Jacob JN, Ridge R, Campbell A, Baldessarini RJ (1984) Gamma aminobutyric acid esters-synthesis, brain uptake and pharmacological studies of aliphatic and steroid esters of gamma aminobutyric acid. J Med Chem 27:659–664
Simpkins JW, Bodor N, Enz A (1985) Direct evidence for brain-specific release of dopamine from a redox delivery system. J Pharm Sci 74:1033 -1036
Tejani-Butt SM, Brunswick DJ (1986) Synthesis and beta-adrenergic receptor blocking potency of 1-(substituted-amino)-3-(4-indolyloxy propan-2-ols). J Med Chem 29:1524–1527
Westerink BHC, Mulder TBA (1981) Determination of picomole amounts of dopamine, noradrenaline, 3,4-dihydroxyphenylalanine, 3,4-dihydroxyphenylacetic acid, homovanillic acid and 5-hydroxyindoleacetic acid in nervous tissue after one-step purification on Sephadex G10, using high-performance liquid chromatography with a novel type of electrochemical detection. J Neurochem 36:1449–1462
Author information
Authors and Affiliations
Additional information
Send offprint requests to S. M. Tejani-Butt at the above address
Rights and permissions
About this article
Cite this article
Tejani-Butt, S.M., Hauptmann, M., D'Mello, A. et al. Evaluation of mono- and dibenzoyl esters of dopamine as potential pro-drugs for dopamine in the central nervous system. Naunyn-Schmiedeberg's Arch Pharmacol 338, 497–503 (1988). https://doi.org/10.1007/BF00179320
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00179320