Summary
Balb/c × DBA/2 F1 mice (CD2F1 mice) bearing L1210 lymphatic leukemia (10 L1210 cells i.p. injected on day 0) were subjected to chemoimmunotherapy. They received 100 mg/kg of cyclophosphamide i.p. on day + 8 and 106 or 107 immunogenic L1210 cells treated in vitro with mafosfamide — ASTA Z7654 (L1210-Maf cells) i.p. or i.p. + s.c. on days 0, + 3, + 6, + 9, + 12 after the leukemia implantation.
About 30% of leukemia-bearing mice receiving cyclophosphamide and L1210-Maf cells after L1210 inoculation were able to reject the leukemia (as compared with 0% after injection of L1210-Maf cells only or 5% after cyclophosphamide administration). Better results (54% of cured mice) were obtained if 107 L1210-Maf cells were injected i.p. + s.c. beside cyclophosphamide. Biological response modifiers (BRM's): levamisole, BCG, bestatin did not improve these results in the doses used in the experiment.
Augmentation of anti-L1210 therapeutic response is dependent on the administration of cyclophosphamide and L1210-Maf cels. Cyclophosphamide not only reduces the tumor burden but probably can potentiate the L1210-Maf dependent antitumor immunity as well.
Similar content being viewed by others
Reference
Skórski T, Kawalec M: New application of a stabilized active cyclophosphamide derivative (Mafosfamide, ASTA Z7654) — immunogenic properties of lymphatic leukemia L1210 cells treated in vitro with the drug. Invest New Drugs 5: 167–169, 1987
Kawalec M, Jakóbisiak M, Skórski T, Kawiak J: Immunogenicity of cyclophosphamide-treated leukemia cells. Folia Biol (Praha) 28:334–343, 1982
Skórski T, Kawalec M, Hoser G: Cyclophosphamide or ifosfamide treated L1210 leukemia cells: immunogenicity, viability and metabolism. Folia Biol (Praha) 32:354–365, 1986
Kawalec M, Skórski T, Hoser G: Immunogenicity of cyclophosphamide-treated tumor cells. Folia Histochem Cytobiol 26: in press, 1988
Skórski T, Kawalec M, Kawiak J: Mechanisms of immunological responses induced in CD2F1, mice by administration of semisyngeneic L1210 leukemia cells treated with cyclophosphamide. Immunol Invest 16:33–43, 1987
Skórski T, Kawalec M, Kawiak J: Cellular composition of spleen and peritoneal exudate in mice after injection of cyclophosphamide-treated L1210 leukemia cells. Folia Histochem Cytobiol 25:23–28, 1987
Lichtfield JT: A method for rapid graphic solution of time-percent effect curves. J Pharmacol Exp Ther 97:399–408, 1949
Lichtfield JT, Wilcoxon F: A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96:99–113, 1949
Gomi K, Murimoto M, Kataoka T: Effect of levamisole on cytotoxic T-cell-mediated immune resistance to L1210 murine leukemia in hyperimmune mice. Cancer Immunol Immunother 17:195–199, 1984
Symoens J, Rosenthal M, De Brabander M, Goldstein G: Immunoregulation with Levamisole. In: L Chedid, PA Miescher, HI Mueller-Eberhard (eds) Immunostimulation. Springer-Verlag, Berlin, Heidelberg, New York, 1980, pp 195–214
Mathe G, Halle-Panenko O, Bourut C: Immune manipulation by BCG administered before or after cyclophosphamide for chemoimmunotherapy of L1210 leukemia. Eur J Cancer 10:661–669, 1974
Talmadge JE, Lenz BF, Pennington R, Long C, Phillips H, Schneider M, Tribble H: Immunodulatory and therapeutic properties of bestatin in mice. Cancer Res 46:4505–4510, 1986
Abe F, Shibuya K, Uchida M, Takahashi K, Horinishi H, Matsuda A, Ishizuka M, Takeuchi T, Umezawa H: Effect of bestatin on syngeneic tumors in mice. Gann 75:89–94, 1984
Gershwin ME, Goetel EJ, Steinberg AD: Cyclophosphamide: use in practice. Ann Int Med 80:531–540, 1974
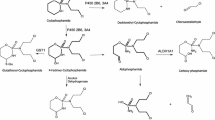
Alberts DS, Einspahr JG, Struck R, Bignami G, Young L, Surwit EA, Salmon SE: Comparative in vitro cytotoxicity of cyclophosphamide, its major active metabolites and the new oxazaphosphorine ASTA Z 7557 (INN mafosfamide). Invest New Drugs 2: 141–148, 1984
Niemeyer U, Engel J, Scheffler G, Molge K, Sauerbier D, Weigert W: Chemical characterization of ASTA Z 7557 (INN mafosfamide, CIS-4-sulfoethylthiocyclophosphamide), a stable derivative of 4-hydroxy-cyclophosphamide. Invest New Drugs 2:133–139, 1984
Santos GW, Colvin OM: Pharmacological purging of bone marrow with reference to autografting. Clin Haematol 15:67–83, 1986
Berd D, Maguire HC, Mastrangelo MJ: Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Research 46:2572–2577, 1986
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawalec, M., Skórski, T. & Kawiak, J. Successful chemoimmunotherapy of murine L1210 lymphatic leukemia with cyclophosphamide and mafosfamide-treated leukemia cells. Invest New Drugs 6, 169–172 (1988). https://doi.org/10.1007/BF00175393
Issue Date:
DOI: https://doi.org/10.1007/BF00175393