Summary
Twenty-nine patients with advanced ovarian carcinoma were entered into a Gynecologic Group Phase II study of Baker's Antifol. Of these, 26 were eligible for evaluation of toxicity and 25 for evaluation of response. The evaluable patients constituted an unusually favorable group for a Phase II study in a chemotherapy-sensitive tumor; although all have received prior chemotherapy, eight had had treatment with only a single alkylating agent and the median performance status of the study population was two (ambulatory, capable of self-care).
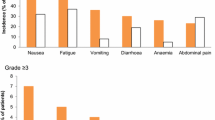
No complete responses were seen. Two patients had regression of abdominal tumor masses sufficient to qualify as partial responders (PR 8%). Dose-limiting toxicity, as expected, was found to be gastrointestinal. Significant mucositis and dermatitis were also observed. No episodes of hypotension during infusion occurred with a 60–120 min time of administration.
Baker's antifol has only limited activity against ovarian carcinoma previously treated with chemotherapy and is not likely to contribute to improved therapy, either as a single agent or in combination.
Similar content being viewed by others
References
Vogl SE, Pagano M, Kaplan BH: Cyclophosphamide, hexamethylmelamine, adriamycin, and diamminedichloroplatinum — “CHAD” vs. melphalan for advanced ovarian carcinoma — a randomized prospective trial of the Eastern Cooperative Oncology Group. Proc Amer Assoc Cancer Res and ASCO 22:429, 1981
Omura GA, Ehrlich CE, Blessing JA: A randomized trial of cyclophosphamide plus adriamycin with or without cisplatinum in ovarian cancer. Proc Amer Soc Clin Oncol 1:104, 1982
Baker BR: Active site-directed irreversible inhibitors of dihydrofolate reductase. Ann New York Acad Sci 186:214–226, 1971
Skeel RT, Sawicki WL, Cashmore AR, Bertino JR: Basis for the disparate sensitivity of L1210 leukemia and Walker 256 carcinoma to a new triazine folate antagonist. Cancer Res 33:2971–2976, 1973
Rodriquez V, Gottlieb J, Burgess MA: Phase I studies with Baker's antifol (BA) (NSC 139, 105). Cancer 38:690–694, 1976
Rodriquez V, Richman SP, Benjamin RS: Phase II study of Baker's antifol in solid tumors. Cancer Res 37:980–983, 1977
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arseneau, J.C., Bundy, B., Homesley, H. et al. Phase II study of Baker's antifol (triazinate, TZT, NSC 139, 105) in advanced carcinoma of the ovary. Invest New Drugs 1, 185–188 (1983). https://doi.org/10.1007/BF00172079
Issue Date:
DOI: https://doi.org/10.1007/BF00172079