Summary
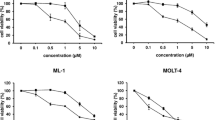
A human tumor cloning system was used to assess the cytotoxicity of adriamycin, mitoxantrone and bisantrene at concentrations that are equitoxic in man. There were 989 specimens evaluable for drug sensitivity analysis. Overall, adriamycin showed in vitro cytotoxicity (≥ 50% decrease in tumor colony forming units) 14% of the time; mitoxantrone, 21% of the time; and bisantrene, 31% of the time. Three hundred ninety-nine of these evaluable specimens were simultaneously tested against more than one of the agents, providing 631 two-way drug comparisons. For these comparisons, there was lack of co-resistance 27–34% of the time, with mitoxantrone being more active than adriamycin (p < .05) and bisantrene being more active than adriamycin (p < .01) or mitoxantrone (p < .01). These data suggest that comparative and sequential clinical use of these agents should be investigated.
Similar content being viewed by others
References
Alberts DS, Chen HSG, Salmon SE: In vitro drug assay: pharmacologic considerations. In Salmon SE (ed): Cloning of Human Tumor Stem Cells. Alan R. Liss, New York, 1980, pp. 197–207
Blum RH, Carter SK: Adriamycin — a new anticancer drug with significant clinical activity. Ann Intern Med 80:249–259, 1974
Citarella RV, Wallace RE, Murdock KC, Angier RB, Durr FE: Antitumor activity of CL216.942: 9,10-Anthracenedi-carboxaldehyde bis ((4,5 dihydro-1H-imidazol-2-yl)-hydrazone)dihydrochloride. Abstracts of the 20th Interscience Conference on Antimicrobial Agents and Chemotherapy, 1980 (Abstract 23)
Hamburger AW, Salmon SE: Primary bioassay of human myeloma stem cells. J Clin Invest 60:846–854, 1977
Hamburger AW, Salmon SE: Primary bioassay of human tumor stem cells. Science 197:461–463, 1977
Hamburger AW, Salmon SE, Kim MB, Trent JM, Soehnlen BJ, Alberts DS, Schmidt HJ: Direct cloning of human ovarian carcinoma cells in agar. Cancer Res 38:3438–3444, 1978
Johnson RK, Zee-Cheng RKY, Lee WW, Acton FM, Henry DW, Cheng CC: Experimental antitumor activity of aminoanthraquinones. Cancer Treat Rep 63:425–539, 1979
Pike BL, Robinson WA: Human bone marrow colony growth in agar-gel. J Cell Physiol 76:77–81, 1970
Salmon SE, Alberts DS, Meyskens FL, Durie BGM, Jones SE, Soehnlen BJ, Young L, Chen HSG, Moon TE: Clinical correlations of in vitro drug sensitivity. In Salmon SE (ed): Cloning of Human Tumor Stem Cells. Alan R. Liss, New York, 1980, pp. 223–245
Van Echo DA, Shulman PN, Ferrari A, Budman D, Markus SD, Wiernik PH: A phase II trial of Mitoxantrone (DHAD, NSC 301739) in adult acute leukemia. Proc Am Soc Clin Oncol 1:132, 1982
Venditti JM: Preclinical drug development: rationale and methods. Sem Oncol 8:349–361, 1981
Von Hoff DD, Pollard E, Kuhn J, Murray E, Coltman CA Jr; Phase I clinical investigation of 1,4-dihydroxy-5,8-bis ((2-(2-hydroxyethylamino)ethyl)amino)-9,10 anthracenedione dihydrochloride (NSC 301739), a new anthracenedione. Cancer Res 40:1516–1518, 1980
Von Hoff DD, Myers JW, Kuhn J, Sandbach J, Pocelinko R, Clark G, Coltman CJR: Phase I clinical investigation of 9–10 anthracenedicarboxaldehyde bis ((4,5-dihydro-1H-imidazol-2-yl)hydrazone) dihydrochloride (CL216,942). Cancer Res 41:3118–3121, 1981
Von Hoff DD, Casper J, Bradley E, Sandbach J, Jones D, Makuch R: Association between human tumor colony forming assay results and response of an individual patient's tumor to chemotherapy. Am J Med 70:1027–1032, 1981
Wallace RE, Murdock KC, Angier RB, Durr FE: Activity of a novel anthracenedione, 1,4-dihydroxy-5,8-bis 2-[(2-hydroxyethyl)aminoethyl amino-9,-10 anthracenedione dihydrochloride, against experimental tumors in mice. Cancer Res 39:1570–1574, 1979
Yap HY, Blumenschien GR, Schell FC, Buzdar AU, Valdivieso M, Bodey GP: Dihydroxyanthracenedione: a promising new drug in the treatment of metastatic breast cancer. Ann Int Med 95:694–697, 1981
Zee-Cheng RKY, Cheng CC: Antineoplastic agents. Structure activity relationship study of bis (substituted aminoalkylamino)anthraquinones. J Med Chem 21:291–294, 1978
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cowan, J.D., Von Hoff, D.D. & Clark, G.M. Comparative cytotoxicity of adriamycin, mitoxantrone and bisantrene as measured by a human tumor cloning system. Invest New Drugs 1, 139–144 (1983). https://doi.org/10.1007/BF00172072
Issue Date:
DOI: https://doi.org/10.1007/BF00172072