Summary
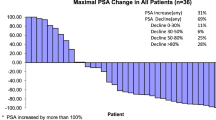
Twenty-nine patients with metastatic prostate cancer progressing after hormonal therapy (orchiectomy 19, diethylstilbesterol 10) and who had never received cytotoxic therapy were treated with carboplatin. Patients had good clinical performance status (66% PS 0,1) and adequate renal (creatinine < 2.0 mg/dL) and bone marrow function. The standard dose of carboplatin administered was 400 mg/sq m. Seventeen patients received this dose and 12 either 320 mg/sq m or 250 mg/sq m based on reduced renal function or prior radiation. Five patients had bidimensionally measurable disease: one experienced a partial regression of cervical lymph node metastases of 97 days duration. Twenty-four patients had metastatic disease evaluable by clinical status, bone scan and acid phosphatase. In one patient > 50% reduction in number of abnormal areas of bone scan uptake occurred; 3 patients experienced improvement in clinical status; in no patient did an elevated prostate acid phosphatase return to normal. All patients entered on study have progressed and died: median time to progression was 94 days (6 to 625 days); median survival was 297 days (6–1152 days). The primary toxicity of carboplatin was myelosuppression. The median WBC and platelet nadirs after cycle one were 3150/cu mm and 93,000/cu mm, respectively. Dose escalations to grade 2 or greater myelosuppression were mandated. Twenty-six achieved at least grade 2 myelosuppression during carboplatin treatment. We conclude that carboplatin administered at this dose and schedule has no important activity in hormone refractory prostate cancer.
Similar content being viewed by others
References
Stanford EJ, Drago JR, Rohner TJ, Santen R, Lipton A: Aminoglutethimide medical adrenalectomy for advanced parostatic carcinoma. J Urol 115: 170–174, 1976
Havlin K, Jordan VC, Cummings K, Messing E, Trump DL: Ketaconazole (KC) in advanced prostate cancer (CaP) refractory to initial hormonal therapy. A clinical and endocrinologic study. Proc Am Soc Clin Oncol 6: 106, 1987
Muss HB, Howard V, Richards F II, White DR, Jackson DV, Cooper R, Stuart JJ, Resnick MI, Brodkin R, Spurr CL: Cyclophosphamide versus cyclophosphamide, methotrexate, and 5-fluorouracil in advanced prostatic cancer: A randomized trial. Cancer 47: 1949–1953, 1981
DeWys W, Begg C, Brodovsky H, Creech R, Khandekar J: A comparative clinical trial of adriamycin and 5-fluorouracil in advanced prostatic cancer: prognostic factors and response. Prostate 4: 1–11, 1983
Scher H, Yagoda A, Watson RC, Serbern M, Whitmore W: Phase II trial of doxorubicin in bidimensionally measurable prostatic adenocarcinoma. J Urol 131: 1099–1122, 1984
Logothetis CJ, Samuels ML, von Eschenbach AC, Trindade A, Ogden S, Grant C, Johnson D: Doxorubicin, mitomycin-C, and 5-fluorouracil (DMF) in the treatment of metastatic- hormonal refractory adenocarcinoma of the prostate, with a note on the staging of metastatic prostate cancer. J Clin Oncol 1: 368–379, 1984
Tannock IF: Is there evidence that chemotherapy is of benefit to patients with carcinoma of the prostate? J CLin Oncol 3: 1013–1021, 1985
Merrin CE, Beckly S: Treatment of estrogen-resistant stageD carcinoma of prostate with cis diamminedichloroplatinum. Urol 13: 267, 1979
Prestayko AW, Bradner WT, Huftalen JB: Antileukemic (L1210) activity and toxicity of cisdiamminedichloroplatinum (II) analogues. Cancer Treat Rep 63: 1503–1508, 1979
Rose WC, Bradner WT: Experimental antitumor activity of platinum coordination complexes. In: Hacker MP, Douple EB, Krakoff IH (eds) Platinum Coordination Complexes in Cancer Chemotherapy, 1984, pp 228–239
Yagoda A, Watson RC, Natale RB et al.: A critical analysis of response criteria in patients with prostatic cancer treated with cis-diamminedichloride platinum II. Cancer 44: 1533–1562, 1979
Author information
Authors and Affiliations
Additional information
Other participating institutions include: New York University Medical Center, New York, NY, CA 16395, USA
Rights and permissions
About this article
Cite this article
Trump, D.L., Marsh, J.C., Kvols, L.K. et al. A phase II trial of carboplatin (NSC 241240) in advanced prostate cancer, refractory to hormonal therapy. Invest New Drugs 8 (Suppl 1), S91–S94 (1990). https://doi.org/10.1007/BF00171992
Issue Date:
DOI: https://doi.org/10.1007/BF00171992