Summary
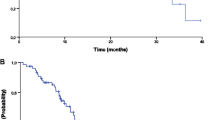
In this phase II trial, menogaril was administered to patients with metastatic colon cancer at a dose of 200 mg/m2 IV over one hour with cycles repeated every 28 days provided the absolute granulocyte count was ≥ 2000 cells/μl. Dose adjustments up or down were made depending upon nadir counts. Twenty-four patients were entered on this study with 21 eligible and evaluable for response. There was 1 CR lasting four and one-half months and 1 PR lasting three months for an overall CR \s+ PR rate of 10% with a 95% confidence interval of 1% to 30%. Six patients (29%) had stable disease and 13 (62%) progressed. Median survival is 13.1 months. Toxicity was primarily hematologic with two cases of life-threatening leukopenia (< 1000 cells/μl) and one case of life-threatening granulocytopenia (< 250 cells/μl) among the 21 eligible patients, and one case of life-threatening leukopenia and granulocytopenia in one ineligible patient. There were no deaths due to treatment.
Similar content being viewed by others
References
Weiss GR, Bhuyan BK, Kisner DL, Von Hoff DD: 7 (R)-O-Methylnogarol (MEN): antitumor activity in the human tumor cloning system. Invest New Drugs 2: 118, 1984
Neil GL, Kuentzel SL, McGovren JP: Treatment of mouse tumors with 7-con-O-Methylnogarol and other analogs of the anthracycline antibiotics, nogalamycin. Cancer Treat Rep 63: 1971–1978, 1979
Krueger WC, Pschigoda LM, Schpok SLF, Moscowitz A, McGovren JP, Neta P, Merritt MV, Li LH: The interaction of nogalamycin and analogs with DNA and other biopolymers. Chem-Biol Inter 36: 1–18, 1981
Li LH, Kuentzel SL, Murch LL, Pschigoda LM, Krueger WC: Comparative biological and biochemical effects of nogalamycin and its analogs on L1210 Leukemia. Cancer Res 39: 4816–4822, 1979
Bhuyan BK, Blowers CL, Shugars KD: Lethality of nogalamycin, nogalamycin analogs, and Adriamycin to cells in different cell cycle phases. Cancer Res 40: 3437–3442, 1980
Carter SK, Friedman M: Intregration of chemotherapy into combined modality treatment of solid tumors. II. Large bowel carcinoma. Cancer Treat Rev 1: 111–129, 1974
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Whitehead, R.P., Earhart, R.H., Fleming, T. et al. A phase II study of menogaril (NSC-269148) in colorectal carcinoma. Invest New Drugs 8, 295–297 (1990). https://doi.org/10.1007/BF00171840
Issue Date:
DOI: https://doi.org/10.1007/BF00171840