Summary
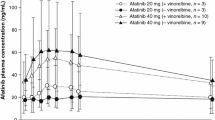
Vinzolidine (VZL) is a semisynthetic vinca alkaloid with broad antitumor activity in animal models of malignancy but had unpredictable toxic effects when given orally to humans. To minimize the toxic effects due to potential erratic gastrointestinal absorption, this drug was restudied in man as an intravenous preparation given as a rapid injection every two weeks. The maximum tolerated dose (MTD) on this schedule was 9.0 mg/m2 with unpredictable leukopenia (usually occurring 5–14 days post treatment but appearing erratically), constipation, paralytic ileus, and inappropriate ADH syndrome as major toxicities. Nonhematologic toxicities were dose-limiting. Repetitive dosing at two week intervals was associated with leukopenia at D 14–15 in some but not all patients treated above 5.0 mg/m2 precluding further treatment on schedule. In contrast, the oral MTD of this agent in our prior studies was 45 mg/m2 with no evidence of delayed leukopenia. Intrapatient variability of toxicity was small; interpatient variability of toxicity was substantial and did not correlate with prior therapy. Because of the presence of delayed hematologic toxicity on repetitive dosing schedules, intravenous VZL should be given on a dosing schedule longer than 14 days. No antitumor activity was seen in this study.
Similar content being viewed by others
References
Vinzolidine. An Investigational New Drug Brochure. Eli Lilly Co. 1981, p 92, on file with the FDA
Budman DR, Schulman P, Marks M, Vinciguerra V, Weiselberg L, Kreis W, Degnan TJ: Phase I trial of Vinzolidine. Can Treat Rep 68: 979–982, 1984
Nelson R: Vinzolidine — A new vinca derivative in clinical trials (Abstr), Chemotherapy Foundation Symposium IV, pp 65–66, 1984
Koeller JM, Trump DL, Witte RS, Davis TE, Nelson RL, Tormey DC, Ramirez G: Phase I trial and pharmacokinetics of Vinzolidine on a daily × 5 oral schedule (Abstr). Proc Am Assoc Cancer Res 25: 643a, 1984
Takasugi BJ, Robertone AB, Salmon SE, Jones SE, Alberts DS: Five-day schedule of Vinzolidine, an oral vinca alkaloid, in a variety of tumors. Invest New Drugs 2: 387–390, 1984
Sarna G, Mitsuyasu R, Figlin R, Ambersley J, Groopman J: Oral Vinzolidine as therapy for Kaposi's sarcoma and carcinomas of lung, breast, and colon/rectum. Cancer Chemother Pharmacol 14: 12–14, 1985
Miller AB, Hoogstraten B, Staquet M, Winkler A: Reporting results of cancer treatment. Cancer 47: 207–214, 1981
Kreis W, Budman DR, Schulman P, Freeman J, Greist A, Nelson RL, Marks M, Kevill L: Clinical pharmacology of vinzolidine. Cancer Chemother Pharmacol 16: 70–74, 1986
Taylor C, Salmon S, Alberts D, Robertone A, Peng Y: Phase I study of intravenous Vinzolidine (Abstr). Proc Am Soc Clin Oncol 6: 81a, 1987
Bissery MC, White K, Polin L, Puzycki D, Zalupski MM: Murine solid tumor activity of Vinzolidine (Abstr). Proc Am Assoc Cancer Res 29:1325a, 1988
Kreis W, Budman DR, Freeman J, Milazzo J, Bergstrom RF, Nelson R: Clinical pharmacology studies with IV administered 3H-Vinzolidine (Abstr). Proc Am Assoc Cancer Res 29:858a, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Budman, D.R., Kreis, W., Behr, J. et al. Phase I trial of intravenous vinzolidine (LY 104208) given on a biweekly dosing schedule. Invest New Drugs 8, 269–274 (1990). https://doi.org/10.1007/BF00171836
Issue Date:
DOI: https://doi.org/10.1007/BF00171836