Abstract
Purpose of Review
To overview recent understanding of the relationship between the blood coagulation cascade and chronic spontaneous urticaria (CSU).
Recent Findings
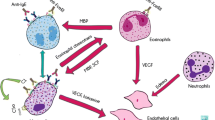
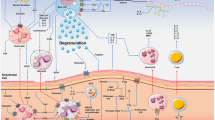
The relationship between severities of CSU and the increase of coagulation markers, and the effectiveness of anti-coagulants, such as warfarin, suggest a causative role of the blood coagulation in the pathogenesis of CSU. However, mechanisms of the initiation of blood coagulation, and the link between blood coagulation and wheal formation in urticaria, remained unclear. In blood vessels, vascular endothelial cells and eosinophils may express tissue factor (TF), which triggers the activation of extrinsic coagulation cascade. We recently revealed that histamine and TLR agonists synergistically induce TF expression by endothelial cells and produce active forms of coagulation factors, such as Xa and IIa, which may induce plasma extravasation. The exposure of skin mast cells to the exuded plasma may then induce degranulation of the skin mast cells via various receptors, releasing a massive amount of histamine, resulting in wheal formation observed in CSU.
Summary
Further elucidation of the mechanism of blood coagulation and the pathway of mast cell activation by activated coagulation factors may be a target for the development of new and more effective treatments for CSU.

Similar content being viewed by others
Abbreviations
- CSU:
-
Chronic spontaneous urticarial
- CIU:
-
Chronic idiopathic urticarial
- TF:
-
Tissue factor
- PAR:
-
Protease-activated receptor
- FcεRI:
-
High-affinity IgE receptor
- HUVEC:
-
Human umbilical vein endothelial cells
- HMVEC:
-
Human dermal microvascular endothelial cells
- TLR:
-
Toll-like receptor
- LPS:
-
Lipopolysaccharide
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharides
- SP:
-
Substance P
- MRGX2:
-
MAS-related G protein-coupled receptor member X2
- NMU:
-
Neuromedin U
- MBP:
-
Major basic protein
- EPO:
-
Eosinophil peroxidase
- CRP:
-
C-reactive protein
- PAF:
-
Platelet-activating factor
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jain S. Pathogenesis of chronic urticaria: an overview. Dermatol Res Pract. 2014 2014; 674,709.
Maurer M, Weller K, Bindslev-Jensen C, Gimenez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66(3):317–30.
Maurer M, Church MK, Gonçalo M, Sussman G, Sánchez-Borges M. Management and treatment of chronic urticaria (CU). J Eur Acad Dermatol Venereol. 2015;29:16–32.
Termeer C, Staubach P, Kurzen H, Strömer K, Ostendorf R, Maurer M. Chronic spontaneous urticaria—a management pathway for patients with chronic spontaneous urticaria. J Dtsch Dermatol Ges. 2015;13(5):419–28.
Harvima IT, Levi-Schaffer F, Draber P, Friedman S, Polakovicova I, Gibbs BF, et al. Molecular targets on mast cells and basophils for novel therapies. J Allergy Clin Immunol. 2014;134(3):530–44.
Hide M, Hiragun M, Hiragun T. Diagnostic tests for urticaria. Immunol Allergy Clin North Am. 2014;34(1):53–72.
Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328(22):1599–604.
Maurer M, Rosén K, Hsieh HJ, Saini S, Grattan C, Gimenéz-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368(10):924–35.
Hatada Y, Kashiwakura J, Hayama K, Fujisawa D, Sasaki-Sakamoto T, Terui T, et al. Significantly high levels of anti-dsDNA immunoglobulin E in sera and the ability of dsDNA to induce the degranulation of basophils from chronic urticaria patients. Int Arch Allergy Immunol. 2013;161(Suppl 2):154–8.
Schmetzer O, Lakin E, Topal FA, Preusse P, Freier D, Church MK, Maurer M. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. In press. Doi: https://doi.org/10.1016/j.jaci.2017.10.035.
Altrichter S, Hawro T, Liedtke M, Holtappels G, Bachert C, Skov PS, et al. In chronic spontaneous urticaria, IgE against staphylococcal enterotoxins is common and functional. Allergy. 2018;73(7):1497–504.
Metz M, Staubach P, Bauer A, Brehler R, Gericke J, Kangas M, et al. Clinical efficacy of omalizumab in chronic spontaneous urticaria is associated with a reduction of FcεRI-positive cells in the skin. Theranostics. 2017;7(5):1266–76.
Larenas-Linnemann DES, Parisi CAS, Ritchie C, Cardona-Villa R, Cherrez-Ojeda I, Cherrez A, et al. Update on omalizumab for urticaria: what’s new in the literature from mechanisms to clinic. Curr Allergy Asthma Rep. 2018;18(5):33.
Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol. 2004;4(5):387–96.
Atwa MA, Emara AS, Youssef N, Bayoumy NM. Serum concentration of IL-17, IL-23 and TNF-α among patients with chronic spontaneous urticaria: association with disease activity and autologous serum skin test. J Eur Acad Dermatol Venereol. 2014;28(4):469–74.
Kasperska-Zajac A, Sztylc J, Machura E, Jop G. Plasma IL-6 concentration correlates with clinical disease activity and serum C-reactive protein concentration in chronic urticaria patients. Clin Exp Allergy. 2011;41(10):1386–91.
•• Kolkhir P, André F, Church MK, Maurer M, Metz M. Potential blood biomarkers in chronic spontaneous urticaria. Clin Exp Allergy. 2017;47(1):19–36 This is a review of various biomarkers involved in the pathogenesis of CSU.
Metz M, Krull C, Hawro T, Saluja R, Groffik A, Stanger C, et al. Substance P is upregulated in the serum of patients with chronic spontaneous urticaria. J Invest Dermatol. 2014;134(11):2833–6.
Kolkhir P, Altrichter S, Hawro T, Maurer M. C-reactive protein is linked to disease activity, impact, and response to treatment in patients with chronic spontaneous urticaria. Allergy. 2018;73(4):940–8.
Lin W, Zhou Q, Liu C, Ying M, Xu S. Increased plasma IL-17, IL-31, and IL-33 levels in chronic spontaneous urticaria. Sci Rep. 2017;7(1):17797.
Rather S, Keen A, Sajad P. Serum levels of 25-hydroxyvitamin D in chronic urticaria and its association with disease activity: a case control study. Indian Dermatol Online J. 2018;9(3):170–4.
Chen T, Fu LX, Sun QM, Zhou PM, Guo ZP. Decreased interleukin-35 serum levels in patients with chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2018: S1081–1206(18)30462–9.
Asero R. Plasma D-dimer levels and clinical response to ciclosporin in severe chronic spontaneous urticaria. J Allergy Clin Immunol. 2015;135(5):1401–3.
Cugno M, Marzano AV, Asero R, Tedeschi A. Activation of blood coagulation in chronic urticaria: pathophysiological and clinical implications. Intern Emerg Med. 2010;5(2):97–101.
Asero R, Tedeschi A, Coppola R, Griffini S, Paparella P, Riboldi P, et al. Activation of the tissue factor pathway of blood coagulation in patients with chronic urticaria. J Allergy Clin Immunol. 2007;119(3):705–10.
Sakurai Y, Morioke S, Takeda T, Takahagi S, Hide M, Shima M. Increased thrombin generation potential in patients with chronic spontaneous urticaria. Allergol Int. 2015;64(1):96–8.
Takeda T, Sakurai Y, Takahagi S, Kato J, Yoshida K, Yoshioka A, et al. Increase of coagulation potential in chronic spontaneous urticaria. Allergy. 2011;66(3):428–33.
Takahagi S, Shindo H, Watanabe M, Kameyoshi Y, Hide M. Refractory chronic urticaria treated effectively with the protease inhibitors, nafamostat mesilate and camostat mesilate. Acta Derm Venereol. 2010;90(4):425–6.
Yanase Y, Takahagi S, Hide M. Chronic spontaneous urticaria and the extrinsic coagulation system. Allergol Int. 2018;67(2):191–4.
Meyer-De Schmid JJ, Neuman A. Treatment of chronic urticaria with heparin. Bull Soc Fr Dermatol Syphiligr. 1952;59(3):286–7.
Chua SL, Gibbs S. Chronic urticaria responding to subcutaneous heparin sodium. Br J Dermatol. 2005;153(1):216–7.
Parslew R, Pryce D, Ashworth J, Friedmann PS. Warfarin treatment of chronic idiopathic urticaria and angio-oedema. Clin Exp Allergy. 2000;30(8):1161–5.
Takahagi S, Mihara S, Iwamoto K, Morioke S, Okabe T, Kameyoshi Y, et al. Coagulation/fibrinolysis and inflammation markers are associated with disease activity in patients with chronic urticaria. Allergy. 2010;65(5):649–56.
Mackman N. The many faces of tissue factor. J Thromb Haemost. 2009;7(Suppl 1):136–9.
Daubie V, Pochet R, Houard S, Philippart P. Tissue factor: a mini-review. J Tissue Eng Regen Med. 2007;1(3):161–9.
Chu AJ. Tissue factor mediates inflammation. Arch Biochem Biophys. 2005;440(2):123–32.
Chu AJ. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam. 2011;2011:367284.
Moosbauer C, Morgenstern E, Cuvelier SL, Manukyan D, Bidzhekov K, Albrecht S, et al. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood. 2007;109(3):995–1002.
Cugno M, Marzano AV, Tedeschi A, Fanoni D, Venegoni L, Asero R. Expression of tissue factor by eosinophils in patients with chronic urticaria. Int Arch Allergy Immunol. 2009;148(2):170–4.
Puccetti A, Bason C, Simeoni S, Millo E, Tinazzi E, Beri R, et al. In chronic idiopathic urticaria autoantibodies against Fc epsilonRII/CD23 induce histamine release via eosinophil activation. Clin Exp Allergy. 2005;35(12):1599–607.
Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. 2016;138(3):700–10.
Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38(4):709–25.
Østerud B. Tissue factor expression in blood cells. Thromb Res. 2010;125(Suppl 1):S31–4.
Siddiqui FA, Desai H, Amirkhosravi A, Amaya M, Francis JL. The presence and release of tissue factor from human platelets. Platelets. 2002;13(4):247–53.
•• Yanase Y, Morioke S, Iwamoto K, Takahagi S, Uchida K, Kawaguchi T, et al. Histamine and TLR ligands synergistically induce endothelial-cell gap-formation by the extrinsic coagulating pathway. J Allergy Clin Immunol. 2018;141(3):1115–8 This study reported synergistic expression of TF on the surface of vascular endothelial cells in response to histamine and LPS.
Vonakis BM, Saini SS. New concepts in chronic urticaria. Curr Opin Immunol. 2008;20(6):709–16.
Zhan M, Zheng W, Jiang Q, Zhao Z, Wang Z, Wang J, et al. Upregulated expression of substance P (SP) and NK1R in eczema and SP-induced mast cell accumulation. Cell Biol Toxicol. 2017;33(4):389–405.
Steffel J, Akhmedov A, Greutert H, Lüscher TF, Tanner FC. Histamine induces tissue factor expression: implications for acute coronary syndromes. Circulation. 2005;112:341–9.
Zucker S, Mirza H, Conner CE, Lorenz AF, Drews MH, Bahou WF, et al. Vascular endothelial growth factor induces tissue factor and matrix metalloproteinase production in endothelial cells: conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int J Cancer. 1998;75(5):780–6.
Del Turco S, Basta G, Lazzerini G, Chancharme L, Lerond L, De Caterina R. Involvement of the TP receptor in TNF-α-induced endothelial tissue factor expression. Vascul Pharmacol. 2014;62(2):49–56.
Stojkovic S, Kaun C, Basilio J, Rauscher S, Hell L, Krychtiuk KA, et al. Tissue factor is induced by interleukin-33 in human endothelial cells: a new link between coagulation and inflammation. Sci Rep. 2016;6:25171.
Scholl A, Ivanov I, Hinz B. Inhibition of interleukin-1β-induced endothelial tissue factor expression by the synthetic cannabinoid WIN 55,212-2. Oncotarget. 2016;7(38):61438–61,457.
Lwaleed BA, Bass PS. Tissue factor pathway inhibitor: structure, biology and involvement in disease. J Pathol. 2006;208(3):327–39.
Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 2014;134(3):622–33.
• Matsuo Y, Yanase Y, Irifuku R, Takahagi S, Mihara S, Ishii K, Kawaguchi T, Tanaka A, Iwamoto K, Watanuki H, Furuta K, Tanaka S, Inoue A, Aoki J, Hide M. Neuromedin U directly induces degranulation of skin mast cells, presumably via MRGPRX2. Allergy. In press. https://doi.org/10.1111/all.13555. This study reported the role of NMU, neuropeptide, for the activation of human skin mast cells.
Bossi F, Frossi B, Radillo O, Cugno M, Tedeschi A, Riboldi P, et al. Mast cells are critically involved in serum-mediated vascular leakage in chronic urticaria beyond high-affinity IgE receptor stimulation. Allergy. 2011;66(12):1538–45.
Cugno M, Tedeschi A, Frossi B, Bossi F, Marzano AV, Asero R. Detection of low-molecular-weight mast cell-activating factors in serum from patients with chronic spontaneous urticaria. J Investig Allergol Clin Immunol. 2016;26(5):310–3.
He SH, Xie H, Fu YL. Activation of human tonsil and skin mast cells by agonists of proteinase activated receptor-2. Acta Pharmacol Sin. 2005;26(5):568–74.
Moormann C, Artuc M, Pohl E, Varga G, Buddenkotte J, Vergnolle N, et al. Functional characterization and expression analysis of the proteinase-activated receptor-2 in human cutaneous mast cells. J Invest Dermatol. 2006;126(4):746–55.
Carvalho RF, Nilsson G, Harvima IT. Increased mast cell expression of PAR-2 in skin inflammatory diseases and release of IL-8 upon PAR-2 activation. Exp Dermato.l. 2010;19(2):117–22.
Vliagoftis H. Thrombin induces mast cell adhesion to fibronectin: evidence for involvement of protease-activated receptor-1. J Immunol. 2002;169(8):4551–8.
Asero R, Riboldi P, Tedeschi A, Cugno M, Meroni P. Chronic urticaria: a disease at a crossroad between autoimmunity and coagulation. Autoimmun Rev. 2007;7(1):71–6.
Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–7.
Ali H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol Lett. 2010;128(1):36–45.
Razin E, Marx G. Thrombin-induced degranulation of cultured bone marrow-derived mast cells. J Immunol. 1984;133(6):3282–5.
• Matsuo Y, Yanase Y, Irifuku R, Ishii K, Kawaguchi T, Takahagi S, Hide I, Hide M. The role of adenosine for IgE receptor-dependent degranulation of human peripheral basophils and skin mast cells. Allergol Int. In press. https://doi.org/10.1016/j.alit.2018.03.007. This study reported the suppressive effect of adenosine on IgE receptor-dependent activation of human skin mast cells and basophils.
Grattan CE and Marsland AM: Chapter 42 Urticaria. In; Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D. Edotors. Rook’s textbook of dermatology. John Wiley & Sons, Ltd., West Sussex, UK; 2016.
Funding
The study was funded by grants to Y.Y from Takeda Science Foundation 2018 and Grant-in-Aid for Scientific Research (C).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yuhki Yanase declares that he has no conflict of interest. Shunsuke Takahagi declares that he has no conflict of interest. Michihiro Hide declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Urticaria and Atopic Dermatitis
Rights and permissions
About this article
Cite this article
Yanase, Y., Takahagi, S. & Hide, M. Role of TF-Triggered Activation of the Coagulation Cascade in the Pathogenesis of Chronic Spontaneous Urticaria. Curr Treat Options Allergy 5, 383–391 (2018). https://doi.org/10.1007/s40521-018-0183-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-018-0183-3