Abstract
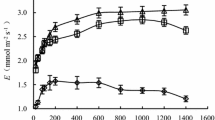
The adaption of photosynthesis, being a key metabolic process, plays an important role in plant resistance to air drought. In this study, the Siberian crabapple (Malus baccata L. Borkh.) in the forest-steppe zone of Transbaikalia region, Russia, was subjected to air drought stress and its photosynthesis characteristics were analyzed. The results show that air drought and sufficient soil moisture supply lead to the decrease in the total chlorophyll (Chl) content, while the ratio of Chls to carotenoids is constant in the Siberian crabapple tree. The function of photosystem II (PS-II) in the crabapple trees is characterized by a decrease in the fraction of absorbed light energy spent on the photochemical work and an increase in the proportion of non-photosynthetic thermal quenching. These changes indicate the photosynthetic down-regulation that acts as a universal photoprotective mechanism. During the midday hours, the combination of high air temperature and low air humidity leads to the decrease in the maximum photochemical quantum yield of photosystem II (Fv/Fm) and the efficiency of photosynthesis (PABS). The parameters of leaf gas exchange show the significant differences in these values between the control and experimental variants. During the morning hours, the Siberian crabapple, growing in the Irkutsk City, assimilates carbon dioxide more intensively. Due to the higher air humidity, the stomata are kept open and the necessary amount of carbon dioxide entries the sites of carboxylation. The low air humidity combined with wind in the experimental variants leads to the unreasonably high water loss in the crabapple leaves by more than 27% as compared to the control variant (Irkutsk City). However, water use efficiency in the morning hours increases during plant photosynthetic processes, i.e., 42% higher than that of control. This, apparently, is a reflection of the adaptation processes of the Siberian crabapple to the air drought and parching wind.
Similar content being viewed by others
Abbreviations
- ABS:
-
absorbed energy flux
- Chl:
-
chlorophyll
- ET:
-
electron transport flux
- ETR:
-
electron transport rate
- F0 :
-
minimum fluorescence yield in dark-adapted state
- Fm :
-
maximum fluorescence yield in dark-adapted state
- Fm′:
-
maximum fluorescence yield in light-adapted state
- F0′:
-
minimum fluorescence yield in light-adapted state
- Fv/Fm :
-
quantum yield of photosystem II
- PAR:
-
photosynthetically active radiation
- PS-II:
-
photosystem II
- RC:
-
reaction center
- TR:
-
trapping flux
- VPD:
-
vapour pressure deficit
- WUE:
-
water use efficiency
- Y(II):
-
effective quantum yield of photosystem II
- Y(NPQ):
-
quantum yield of non-photochemical quenching.
- Ψ0 :
-
probability that a photon trapped by the PS-II reaction center enters the electron transport chain beyond QA (the primary electron acceptor quinone in PS-II) (at t=0)
- φPo :
-
the maximum quantum yield of primary photochemistry at t=0
References
Anenkhonov O A, Korolyuk A Y, Sandanov D V, et al. 2015. Soil-moisture conditions indicated by field-layer plants help identify vulnerable forests in the forest-steppe of semi-arid Southern Siberia. Ecological Indicators, 57: 196–207.
Basu S, Ramegowda V, Kumar A, et al. 2016. Plant adaptation to drought stress. F1000Research, 5(F1000 Faculty Rev): 1554–1563.
Bodner G, Nakhforoosh A, Kaul H-P. 2015. Management of crop water under drought: a review. Agronomy for Sustainable Development, 35(2): 401–442.
Borland A M, Wullschleger S D, Weston D J, et al. 2015. Climate-resilient agroforestry: physiological responses to climate change and engineering of crassulacean acid metabolism (CAM) as a mitigation strategy. Plant, Cell and Environment, 38(9): 1833–1849.
Brestic M, Zivcak M. 2013. PSII fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: protocols and applications. In: Rout G, Das A. Molecular Stress Physiology of Plants. India: Springer, 87–131.
Cruces E, Rautenberger R, Rojas-Lillo Y, et al. 2017. Physiological acclimation of Lessonia spicata to diurnal changing PAR and UV radiation: differential regulation among down-regulation of photochemistry, ROS scavenging activity and phlorotannins as major photoprotective mechanisms. Photosynthesis Research, 131(2): 145–157.
Ghotbi-Ravandi A A, Shahbazi M, Shariati M, et al. 2014. Effects of mild and severe drought stress on photosynthetic efficiency in tolerant and susceptible barley (Hordeum vulgare L.) genotypes. Journal of Agronomy and Crop Science, 200(6): 403–415.
Huang P, Wan X, Lieffers V J. 2016. Daytime and nighttime wind differentially affects hydraulic properties and thigmomorphogenic response of poplar saplings. Physiologia Plantarum, 157(1): 85–94.
Jahns P, Holzwarth A R. 2012. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochimica et Biophysica Acta, 1817(1): 182–193.
Kalaji H M, Schansker G, Ladle R J, et al. 2014. Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynthesis Research, 122(2): 121–158.
Kharuk V I, Ranson K J, Oskorbin P A, et al. 2013. Climate induced birch mortality in Trans-Baikal lake region, Siberia. Forest Ecology and Management, 289: 385–392.
Kullaj E, Avdiu V, Lepaja L, et al. 2017. Modeling canopy transpiration and stomatal conductance of young apples using a parameterized Penman-Monteith equation. Acta Horticulture, 1177: 405–412.
Lang Y, Wang M, Xia J, et al. 2018. Effects of soil drought stress on photosynthetic gas exchange traits and chlorophyll fluorescence in Forsythia suspense. Journal of Forestry Research, 29(1): 45–53.
Law B E. 2015. Regional analysis of drought and heat impacts on forests: current and future science directions. Global Change Biology, 20(12): 3595–3599.
Li Y G, Jiang G M, Niu S L, et al. 2003. Gas exchange and water use efficiency of three native tree species in Hunshandak Sandland of China. Photosynthetica, 41(2): 227–232.
Liang E, Leuschner C, Dulamsuren C, et al. 2016. Global warming-related tree growth decline and mortality on the north-eastern Tibetan plateau. Climatic Change, 134(1–2): 163–176.
Lichtenthaler H K, Babani F. 2004. Light adaptation and senescence of the photosynthetic apparatus. Changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity. In: Papageorgiou G C, Govindjee. Advances in Photosynthesis and Respiration. Dordrecht: Springer, 713–734.
Liu C, Liu Y, Guo K, et al. 2011. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environmental and Experimental Botany, 71(2): 174–183.
Liu H, Yin Y, Wang Q, et al. 2015. Climatic effects on plant species distribution within the forest–steppe ecotone in northern China. Applied Vegetation Science, 18(1): 43–49.
Massonnet C, Costes E, Rambal S, et al. 2007. Stomatal regulation of photosynthesis in apple leaves: evidence for different water-use strategies between two cultivars. Annals of Botany, 100(6): 1347–1356.
McDowell N, Pockman W T, Allen C D, et al. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist, 178(4): 719–739.
Murray F W. 1967. On the computation of saturation vapor pressure. Journal of Applied Meteorology, 6: 203–204.
Osipova S, Permyakov A, Permyakova M, et al. 2015. Regions of the bread wheat D genome associated with variation in key photosynthesis traits and shoot biomass under both well watered and water deficient conditions. Journal of Applied Genetics, 57(2): 151–163.
Perez-Martin A, Flexas J, Ribas-Carbo M, et al. 2009. Interactive effects of soil water deficit and air vapour pressure deficit on mesophyll conductance to CO2 in Vitis. Journal of Experimental Botany, 60(8): 2391–2405.
Rudikovskiy A V, Rudikovskaya E G, Dudareva L V, et al. 2008. Unique and rare forms of Siberian apple tree in Selenga district of Buryatia. Siberian Ecological Journal, 2: 327–333.
Schoppach R, Fleury D, Sinclair T R, et al. 2017. Transpiration sensitivity to evaporative demand across 120 years of breeding of Australian wheat cultivars. Journal of Agronomy and Crop Science, 203(3): 219–226.
Sehgal A, Sita K, Bhandari K, et al. 2018. Influence of drought and heat stress, applied independently or in combination during seed development, on qualitative and quantitative aspects of seeds of lentil (Lens culinaris Medikus) genotypes, differing in drought sensitivity. Plant, Cell and Environment, 42(1): 198–211.
Strasser R J, Tsimilli-Michael M, Srivastava A. 2004. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou G C, Govindjee. Advances in Photosynthesis and Respiration. Dordrecht: Springer, 321–362.
von Wettstein D. 1957. Chlorophyll lethals and submicroscopic morphological changes in plastids. Experimental Cell Research, 12(3): 427–506. (in German)
Zandalinas S I, Mittler R, Balfagon D, et al. 2018. Plant adaptations to the combination of drought and high temperatures. Physiologia Plantarum, 162(1): 2–12.
Zhang S Y, Zhang G C, Gu S Y, et al. 2010. Critical responses of photosynthetic efficiency of goldspur apple tree to soil water variation in semiarid loess hilly area. Photosynthetica, 48(4): 589–595.
Acknowledgements
The research was funded by the Siberian Branch of the Russian Academy of Sciences (Integration Project No. 105). The work was performed at the Siberian Institute of Plant Physiology and Biochemistry, Siberian Branch of the Russian Academy of Sciences (Irkutsk City). We thank Dr. Larisa GARKAVA-GUSTAVSSON for comments on the manuscript and Dr. Alexandra YAZEVA for her work on the translation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rudikovskii, A., Rudikovskaya, E. & Dudareva, L. Impact of air drought on photosynthesis efficiency of the Siberian crabapple (Malus baccata L. Borkh.) in the forest-steppe zone of Transbaikalia, Russia. J. Arid Land 11, 255–266 (2019). https://doi.org/10.1007/s40333-019-0006-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40333-019-0006-9