Abstract
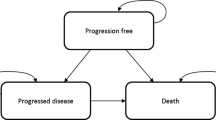
The manufacturer of olaratumab (Lartruvo®), Eli Lilly & Company Limited, submitted evidence for the clinical and cost effectiveness of this drug, in combination with doxorubicin, for untreated advanced soft tissue sarcoma (STS) not amenable to surgery or radiotherapy, as part of the National Institute for Health and Care Excellence (NICE) Single Technology Appraisal process. The Peninsula Technology Assessment Group, commissioned to act as the Evidence Review Group (ERG), critically reviewed the company’s submission. Clinical effectiveness evidence for the company’s analysis was derived from an open-label, randomised controlled trial, JGDG. The analysis was based on a partitioned survival model with a time horizon of 25 years, and the perspective was of the UK National Health Service (NHS) and Personal Social Services. Costs and benefits were discounted at 3.5% per year. Given the available evidence, olaratumab is likely to meet NICE’s end-of-life criteria. To improve the cost effectiveness of olaratumab, the company offered a discount through a Commercial Access Agreement (CAA) with the NHS England. When the discount was applied, the mean base-case and probabilistic incremental cost-effectiveness ratios (ICERs) for olaratumab plus doxorubicin versus the standard-of-care doxorubicin were £46,076 and £47,127 per quality-adjusted life-year (QALY) gained, respectively; the probability of this treatment being cost effective at the willingness-to-pay threshold of £50,000 per QALY gained, applicable to end-of-life treatments, was 0.54. The respective ICERs from the ERG’s analysis were approximately £60,000/QALY gained, and the probability of the treatment being cost effective was 0.21. In August 2017, the NICE Appraisal Committee recommended olaratumab in combination with doxorubicin for this indication for use via the UK Cancer Drugs Fund under the agreed CAA until further evidence being collected in the ongoing phase III trial—ANNOUNCE—becomes available in December 2020.



Similar content being viewed by others
Change history
24 February 2018
Page 41, Column 1, Section 3.1, paragraph 2, 1st sentence which
References
Dangoor A, Seddon B, Gerrand C, Grimer R, Whelan J, Judson I. UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res. 2016;6(1):20.
Cancer Research UK. Soft tissue sarcoma statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/soft-tissue-sarcoma#heading-Zero. Accessed 26 May 2017.
Fletcher C, Sundaram M, Rydholm A, Coindre J, Singer S. WHO classification of soft tissue tumours. 2013. https://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb5/bb5-classifsofttissue.pdf. Accessed 16 Jun 2017.
Costa J, Wesley RA, Glatstein E, Rosenberg SA. The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases. Cancer. 1984;53(3):530–41.
Guillou L, Coindre JM, Bonichon F, Nguyen BB, Terrier P, Collin F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15(1):350–62.
Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33(1):37–42.
National Institute for Health and Care Excellence. Olaratumab in combination with doxorubicin for treating advanced soft tissue sarcoma. 2017. https://www.nice.org.uk/guidance/ta465/evidence. Accessed 9 Aug 2017.
Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–97.
The European Medicines Agency. New treatment for patients with soft tissue sarcoma. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2016/09/WC500212892.pdf. Accessed 26 May 2017.
National Institute for Health and Care Excellence. Guide to the single technology appraisal process. NICE, London. 2009. https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/Guide-to-the-single-technology-appraisal-process.pdf. Accessed 6 Apr 2016.
National Institute for Health and Care Excellence. Olaratumab in combination with doxorubicin for treating advanced soft tissue sarcoma [ID959]. 2016. https://www.nice.org.uk/guidance/indevelopment/gid-ta10103. Accessed Jun 2017.
Seddon BM, Whelan J, Strauss SJ, Leahy MG, Woll PJ, Cowie F et al. GeDDiS: a prospective randomised controlled phase III trial of gemcitabine and docetaxel compared with doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft tissue sarcomas (EudraCT 2009-014907-29) [abstract no. 10500]. J Clin Oncol. 2015;33(15 Suppl).
Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay J-Y, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15(4):415–23.
Santoro A, Tursz T, Mouridsen H, Verweij J, Steward W, Somers R, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13(7):1537–45.
Le Cesne A, Judson I, Crowther D, Rodenhuis S, Keizer H, Van Hoesel Q, et al. Randomized phase III study comparing conventional-dose doxorubicin plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus recombinant human granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: a trial of the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 2000;18(14):2676–84.
Maurel J, López-Pousa A, de las Peñas R, Fra J, Martín J, Cruz J, et al. Efficacy of sequential high-dose doxorubicin and ifosfamide compared with standard-dose doxorubicin in patients with advanced soft tissue sarcoma: an open-label randomized phase II study of the Spanish group for research on sarcomas. J Clin Oncol. 2009;27(11):1893–8.
Guyot P, Ades A, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. 2013. http://www.nice.org.uk/article/pmg9/chapter/foreword.
Mytelka D, Lorenzo M, Stafkey-Mailey D, D’yachkova Y, Nagar S, Candrilli S et al. Real-world treatment patterns and outcomes for patients with advanced soft tissue sarcoma receiving systemic therapy in the United Kingdom. The Ispor Scientific Presentations Database. 2016. https://www.ispor.org/ScientificPresentationsDatabase/Presentation/67700. Accessed 12 Jun 2017.
Woods B, Sideris E, Palmer S, Latimer N, Soares M. NICE DSU TSD19: partitioned survival analysis for decision modelling in health care: a critical review. 2017. http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2017/06/Partitioned-Survival-Analysis-final-report.pdf. Accessed 12 Jun 2017.
National Institute for Health and Care Excellence. Technology Appraisal 176 (TA176): Cetuximab for the first-line treatment of metastatic colorectal cancer. London: National Institute for Health and Care Excellence; 2009.
Van Glabbeke M, Van Oosterom A, Oosterhuis J, Mouridsen H, Crowther D, Somers R, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2185 patients treated with anthracycline-containing first-line regimens: a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17(1):150.
Office of National Statistics. National Life Tables, England, 1980–82 to 2012–2014. 2016. https://www.ons.gov.uk/timeseriestool. Accessed 9 Aug 2017.
Reichardt P, Leahy M, Garcia Del Muro X, Ferrari S, Martin J, Gelderblon H. Quality of life and utility in patients with metastatic soft tissue and bone sarcoma: the Sarcoma Treatment and Burden of Illness in North America and Europe (SABINE) Study. Sarcoma. 2012;2012:740279.
National Institutes of Health. A Study of Doxorubicin Plus Olaratumab (LY3012207) in participants with advanced or metastatic soft tissue sarcoma (ANNOUNCE). 2015. https://clinicaltrials.gov/ct2/show/NCT02451943. Accessed 13 Jun 2017.
National Institute for Health Research. Health Technology Assessment. https://www.nihr.ac.uk/funding-and-support/funding-for-research-studies/funding-programmes/health-technology-assessment/. Accessed 12 Sept 2017.
Acknowledgements
The authors are grateful for the excellent administrative support provided by Mrs. Sue Whiffin and Ms. Jenny Lowe.
Author information
Authors and Affiliations
Contributions
IAT drafted the final version of the manuscript, and TJH, JD, FSW, SR, PS and MH revised the manuscript prior to submission. MH is the overall guarantor of the content. This summary has not been externally reviewed by PharmacoEconomics.
Corresponding author
Ethics declarations
Funding
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (Project Number 16/54/11). Please visit the HTA programme website for further information [26]. This report presents independent research commissioned by the NIHR, and the views and opinions expressed by the authors do not necessarily reflect those of the NHS, NIHR, Medical Research Council, NIHR HTA Programme or the Department of Health.
Conflicts of Interest
Irina A. Tikhonova, Tracey Jones-Hughes, James Dunham, Fiona C. Warren, Sophie Robinson, Peter Stephens and Martin Hoyle have declared no conflicts of interest.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s40273-018-0635-4.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tikhonova, I.A., Jones-Hughes, T., Dunham, J. et al. Olaratumab in Combination with Doxorubicin for the Treatment of Advanced Soft Tissue Sarcoma: An Evidence Review Group Perspective of a National Institute for Health and Care Excellence Single Technology Appraisal. PharmacoEconomics 36, 39–49 (2018). https://doi.org/10.1007/s40273-017-0568-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-017-0568-3