Abstract
Background
Since the 2011 French guidance updates, cholinesterase inhibitors and memantine are considered optional in the management of dementia and leave physicians free to prescribe based on their clinical expertise.
Objectives
The aims of this study were to analyze the influence of these recent guidance updates on the prescription rates of these drugs and to quantify the impact of potential changes on healthcare expenditures.
Methods
Patients over 65 years old from a representative sample of a national administrative claims database, the French national health insurance database, were retrospectively included from 2006 to 2014. Trends of annual prescription rates were tested using adjusted segmented regression analysis. Drug costs with and without prescribers’ behavioral changes were estimated.
Results
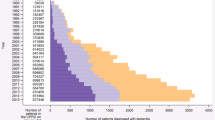
A total of 119,731 individuals were included and followed during the study period. Among them, 5514 individuals were treated for dementia. According to the unadjusted segmented regression model, there was a significant increase in prescription rates between 2006 and 2010, from 2.23% (95% confidence interval 2.13–2.34) to 2.73% (95% confidence interval 2.62–2.84) of the study population. Since 2011, the trend has reversed with a significant decrease until 2014, from 2.64% (95% confidence interval 2.54–2.75) to 1.92% (95% confidence interval 1.84–2.01). In the multivariate analysis, we also found a gradual decline since 2011, particularly for patients aged 65–69 years and with one or more other chronic diseases. Cost savings associated with prescribers’ behavioral changes were estimated at €108 million.
Conclusion
Drugs prescribed for dementia are on a declining trend with important cost savings, and this was concomitant with guidance updates that left physicians to rely on their clinical expertise while managing dementia.



Similar content being viewed by others
References
Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;1:CD005593.
Di Santo SG, Prinelli F, Adorni F, Caltagirone C, Musicco M. A meta-analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer’s disease. J Alzheimers Dis. 2013;35:349–61.
Matsunaga S, Kishi T, Iwata N. Memantine monotherapy for Alzheimer’s disease: a systematic review and meta-analysis. PloS One. 2015;10:e0123289.
Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev. 2012;9:CD009132.
Takeda A, Loveman E, Clegg A, Kirby J, Picot J, Payne E, et al. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:17–28.
Warner J, Butler R. Drugs for Alzheimer’s disease. BMJ. 2001;323:1127.
Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–97.
Fowler NR, Chen Y-F, Thurton CA, Men A, Rodriguez EG, Donohue JM. The impact of Medicare prescription drug coverage on the use of antidementia drugs. BMC Geriatr. 2013;13:37.
Pfeil AM, Kressig RW, Szucs TD. Alzheimer’s dementia: budget impact and cost-utility analysis of a combination treatment of a cholinesterase inhibitor and memantine in Switzerland. Swiss Med Wkly. 2012;142:w13676.
Paraponaris A, Davin B. Economics of the iceberg: informal care provided to French elderly with dementia. Value Health. 2015;18:368–75.
Pariente A, Helmer C, Merliere Y, Moore N, Fourrier-Réglat A, Dartigues J-F. Prevalence of cholinesterase inhibitors in subjects with dementia in Europe. Pharmacoepidemiol Drug Saf. 2008;17:655–60.
Wimo A, Jönsson L, Bond J, Prince M, Winblad B, Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1–11):e3.
Qaseem A, Snow V, Cross JT, Forciea MA, Hopkins R, Shekelle P, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148:370–8.
National Institute for Clinical Excellence. Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease. 2011. Available from: http://www.nice.org.uk/guidance/ta217. Accessed 10 Jun 2015.
Haute Autorité de Santé. Maladie d’Alzheimer et maladies apparentées: diagnostic et prise en charge. 2011. Available from: http://www.has-sante.fr/portail/upload/docs/application/pdf/2011-12/recommandation_maladie_d_alzheimer_et_maladies_apparentees_diagnostic_et_prsie_en_charge.pdf. Accessed 10 Jun 2015.
Sackett DL, Rosenberg WMC, Gray JAM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–2.
Akobeng AK. Principles of evidence based medicine. Arch Dis Child. 2005;90:837–40.
De Roquefeuil L, Studer A, Neumann A, Merlière Y. L’échantillon généraliste de bénéficiaires : représentativité, portée et limites. Prat Organ Soins. 2009;40:213–23.
L’assurance maladie. Les affections de longue durée exonérantes. 2012. Available from: http://www.ameli.fr/professionnels-de-sante/medecins/exercer-au-quotidien/les-affections-de-longue-duree/qu-est-ce-qu-une-affection-de-longue-duree/les-ald-exonerantes.php. Accessed 10 Sep 2015.
Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309.
Zhang F, Wagner AK, Soumerai SB, Ross-Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol. 2009;62:143–8.
Greenhalgh T, Howick J, Maskrey N. Evidence based medicine renaissance group. Evidence based medicine: a movement in crisis? BMJ. 2014;348:g3725.
Stamatakis E, Weiler R, Ioannidis JPA. Undue industry influences that distort healthcare research, strategy, expenditure and practice: a review. Eur J Clin Invest. 2013;43:469–75.
Every-Palmer S, Howick J. How evidence-based medicine is failing due to biased trials and selective publication. J Eval Clin Pract. 2014;20(6):908–14.
Leucht S, Helfer B, Gartlehner G, Davis JM. How effective are common medications: a perspective based on meta-analyses of major drugs. BMC Med. 2015;13:253.
Nicot P. Anticholinestérasiques: le généraliste peut-il dire non ? Médecine. 2010;6:344–6.
Maladie d’Alzheimer et autres démences. Un guide HAS biaisé, des affirmations hasardeuses. La Revue Prescrire. 2009;29:150.
Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? Pharm Ther. 2010;35:208–11.
British Psychological Society and Royal College of Psychiatrists. Dementia: a NICE-SCIE guideline on supporting people with dementia and their carers in health and social care. 2007. Available from: http://www.scie.org.uk/publications/misc/dementia/dementia-fullguideline.pdf?res=true. Accessed 18 Jan 2015.
Jégouzo X, Desbordes M, Delègue S, Le Vacon G, Patrick M, Mouchabac S. Prescription des antipsychotiques chez les sujets âgés avec une démence dans un hôpital psychiatrique: impact des recommandations de l’HAS. L’Encéphale. 2016 Oct 12. doi: 10.1016/j.encep.2016.03.019. (Epub ahead of print).
Pham BN, Musset L, Chyderiotis G, Olsson NO, Fabien N. Celiac disease diagnosis: impact of guidelines on medical prescription in France. J Dig Dis. 2014;15:435–43.
Abraham NS, El-Serag HB, Johnson ML, Hartman C, Richardson P, Ray WA, et al. National adherence to evidence-based guidelines for the prescription of nonsteroidal anti-inflammatory drugs. Gastroenterology. 2005;129:1171–8.
Politi MC, Wolin KY, Légaré F. Implementing clinical practice guidelines about health promotion and disease prevention through shared decision making. J Gen Intern Med. 2013;28:838–44.
Sinnenberg LE, Wanner KJ, Perrone J, Barg FK, Rhodes KV, Meisel ZF. What factors affect physicians’ decisions to prescribe opioids in emergency departments? MDM Policy Pract. 2017;. doi:10.1177/2381468316681006.
HAS (Haute Autorité de Santé). Place des médicaments du traitement symptomatique de la maladie d’Alzheimer. 2012. Available from: http://www.has-sante.fr/portail/upload/docs/application/pdf/2012-03/questions_alzheimer_fiche_bum_mars_2012.pdf. Accessed 18 Jan 2015.
Rabins PV, Rovner BW, Rummans T, Schneider LS, Tariot PN. Guideline watch (October 2014): practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. 2014. Available from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/alzheimerwatch.pdf. Accessed 11 Oct 2015.
Hoffmann F, van den Bussche H, Glaeske G, Kaduszkiewicz H. Eight-year prescription trends of memantine and cholinesterase inhibitors among persons 65 years and older in Germany. Int Clin Psychopharmacol. 2010;25:29–36.
de Hoyos-Alonso MC, Tapias-Merino E, Meseguer Barros CM, Sánchez-Martínez M, Otero A. Consumption trends for specific drugs used to treat dementia in the region of Madrid (Spain) from 2002 to 2012. Neurologia. 2015;30:416–24.
Zilkens RR, Duke J, Horner B, Semmens JB, Bruce DG. Australian population trends and disparities in cholinesterase inhibitor use, 2003 to 2010. Alzheimers Dement. 2014;10:310–8.
Franchi C, Lucca U, Tettamanti M, Riva E, Fortino I, Bortolotti A, et al. Cholinesterase inhibitor use in Alzheimer’s disease: the EPIFARM-Elderly Project. Pharmacoepidemiol Drug Saf. 2011;20:497–505.
Letenneur L, Commenges D, Dartigues JF, Barberger-Gateau P. Incidence of dementia and Alzheimer’s disease in elderly community residents of south-western France. Int J Epidemiol. 1994;23:1256–61.
Franz CE, Barker JC, Kravitz RL, Flores Y, Krishnan S, Hinton L. Nonmedical influences on the use of cholinesterase inhibitors in dementia care. Alzheimer Dis Assoc Disord. 2007;21:241–8.
Mangin D, Heath I, Jamoulle M. Beyond diagnosis: rising to the multimorbidity challenge. BMJ. 2012;344:e3526.
Bunn F, Burn A-M, Goodman C, Rait G, Norton S, Robinson L, et al. Comorbidity and dementia: a scoping review of the literature. BMC Med. 2014;12:192.
Clerc P, Le Breton J. Polyprescription médicamenteuse et polypathologies chroniques: ce qu’en disent les médecins généralistes. Sci Soc Santé. 2013;31:71.
Giron MS, Wang HX, Bernsten C, Thorslund M, Winblad B, Fastbom J. The appropriateness of drug use in an older nondemented and demented population. J Am Geriatr Soc. 2001;49:277–83.
Lau DT, Mercaldo ND, Harris AT, Trittschuh E, Shega J, Weintraub S. Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Dis Assoc Disord. 2010;24:56–63.
Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer’s disease. Arch Med Res. 2012;43:600–8.
Vidal J-S, Lacombe J-M, Dartigues J-F, Pasquier F, Robert P, Tzourio C, et al. Evaluation of the impact of memantine treatment initiation on psychotropics use: a study from the French national health care database. Neuroepidemiology. 2008;31:193–200.
Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, del Ser T, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 2010;30:161–78.
Lenain E, Guen JL, Djadi-Prat J, Somme D, Saint-Jean O, Chatellier G. Identification des sujets atteints d’Alzheimer et autres démences à partir des données de l’Échantillon Généraliste des Bénéficiaires. J Gest Déconomie Médicales. 2014;31:263–71.
Fagnani F, Vespignani H, Kusnik-Joinville O, Bertrand M, Murat C, Lévy-Bachelot L, et al. Intérêt des bases de remboursement de l’Assurance maladie pour l’analyse de la prise en charge des maladies chroniques: le cas de l’épilepsie. Presse Médicale. 2013;42:e285–92.
Tuppin P, Cuerq A, Weill A, Ricordeau P, Allemand H. Maladie d’Alzheimer et autres démences: identification, prise en charge et consommation de neuroleptiques chez les bénéficiaires du régime général (2007–2009). Rev Neurol. 2012;168:152–60.
Lobo A, Launer L, Fratiglioni L, Andersen K, Di Carlo A, Breteler M, et al. Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic diseases in the elderly research group. Neurology. 1999;54:S4–9.
Nowrangi MA, Rao V, Lyketsos CG. Epidemiology, assessment, and treatment of dementia. Psychiatr Clin North Am. 2011;34:275–94.
NICE. Dementia, disability and frailty in later life: mid-life approaches to delay or prevent onset. Guidance and guidelines. Available from: http://www.nice.org.uk/guidance/NG16/chapter/3-Context. Accessed 5 Jan 2016.
Jepsen P, Johnsen SP, Gillman MW, Sørensen HT. Interpretation of observational studies. Heart. 2004;90:956–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the conduct and/or the publication of the research.
Conflict of interest
M François, J. Sicsic, and N. Pelletier-Fleury have no conflicts of interest directly relevant to the content of this study.
Ethics approval
Access to data is subject to prior training and authorization and has received approval from the independent data protection administrative authority (Commission Nationale Informatique et Libertés).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
François, M., Sicsic, J., Elbaz, A. et al. Trends in Drug Prescription Rates for Dementia: An Observational Population-Based Study in France, 2006–2014. Drugs Aging 34, 711–721 (2017). https://doi.org/10.1007/s40266-017-0481-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-017-0481-7