Abstract
Background and Objectives
There is little information on the effects of trandolapril on renal function when used in Canadian general practice. We evaluated the use and blood pressure (BP) lowering effectiveness of trandolapril-based therapies in Canadian conditions of actual care and attempted to capture assessments of urinary albumin concentration (UAC) and estimated glomerular filtration rate (eGFR) in clinical practice.
Methods
This was a prospective, non-interventional, observational study in adults with uncontrolled hypertension, with or without co-morbidities, either treatment-naïve or uncontrolled on existing antihypertensive medications. Hypertension was not defined per protocol. Trandolapril doses (0.5, 1, 2, 4 mg) and subjects’ continued medical care were all at the discretion of the treating physician. Data were gathered after 3, 6 and 12 months.
Results
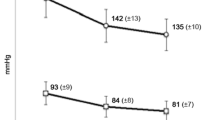
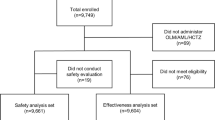
7,993 patients entered the study and 4,983 patients attended the Month 12 visit. Most patients (91.7 %) received trandolapril as a new prescription. At 12 months, 72.9 % of patients without diabetes and 34.4 % with diabetes were controlled (targets <140/90 and 130/80 mmHg, respectively) and 79.2 % of patients with diabetes had BP below 140/90 mmHg. Evaluable eGFR data were available for 25.1, 21.2 and 21.7 % of patients at Months 3, 6 and 12, respectively, and UAC data for 9.6, 8.2 and 9.0 % of patients at the same time points. Treatment was well tolerated. Dropout rates were 37.7 % after 12 months.
Conclusion
Effective, sustained and well-tolerated double-digit BP reduction is achievable with a trandolapril-based treatment regimen for all patient groups. It appears that for diabetic patients blood pressure control as per Canadian Hypertension Education Program recommendations is yet challenging. The results also illuminate the persistent gap between treatment guidelines and actual care.




Similar content being viewed by others
References
Mathers C, Fat DM, Boerma JT, World Health Organization. The global burden of disease 2004 update. Geneva: World Health Organization; 2008. http://site.ebrary.com/id/10266345. Accessed 13 Feb 2012.
Canadian Hypertension Education Program (CHEP). 2012 CHEP Recommendations for Management of Hypertension. 2012. http://hypertension.ca/images/2012_CHEPFullRecommendations_EN_HCP1009.pdf. Accessed 10 Aug 2012.
Strippoli GFM, Craig MC, Schena FP, Craig JC. Role of blood pressure targets and specific antihypertensive agents used to prevent diabetic nephropathy and delay its progression. J Am Soc Nephrol. 2006;17(4 Suppl 2):S153–5.
Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomized controlled trial. Lancet. 2007;370(9590):829–40.
Guay DRP. Trandolapril: a newer angiotensin-converting enzyme inhibitor. Clin Ther. 2003;25(3):713–75.
Tytus RH, Burgess ED, Assouline L, Vanjaka A. Effectiveness of a trandolapril-based treatment regimen in subjects with isolated systolic hypertension in Canada. Curr Med Res Opin. 2009;25(6):1379–84.
Tytus RH, Burgess ED, Assouline L, Vanjaka A. A 26-week, prospective, open-label, uncontrolled, multicenter study to evaluate the effect of an escalating-dose regimen of trandolapril on change in blood pressure in treatment-naive and concurrently treated adult hypertensive subjects (TRAIL). Clin Ther. 2007;29(2):305–15.
Tytus RH, Assouline L, Vanjaka A. Blood pressure control rates with an antihypertensive regimen including trandolapril in a Canadian usual-care setting. Adv Ther. 2011;28(9):789–98.
Backhouse CI, Orofiamma B, Pauly NC. Long-term therapy with trandolapril, a new nonsulfhydryl ACE inhibitor, in hypertension: a multicenter international trial. Investigator Study Group. J Cardiovasc Pharmacol. 1994;23(Suppl 4):S86–90.
Abbott Canada Inc. MAVIK: product monograph. 2011.http://www.abbott.ca. Accessed 5 Aug 2012.
Leenen FHH, Dumais J, McInnis NH, Turton P, Stratychuk L, Nemeth K, et al. Results of the Ontario survey on the prevalence and control of hypertension. CMAJ. 2008;178(11):1441–9.
Thompson SK. Sampling. New York: Wiley; 2002.
Ruilope LM, Zanchetti A, Julius S, McInnes GT, Segura J, Stolt P, et al. Prediction of cardiovascular outcome by estimated glomerular filtration rate and estimated creatinine clearance in the high-risk hypertension population of the VALUE trial. J Hypertens. 2007;25(7):1473–9.
Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003.
Leoncini G, Viazzi F, Pontremoli R. Overall health assessment: a renal perspective. Lancet. 2010;375(9731):2053–4.
Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81.
Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63(1):225–32.
Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157(13):1413–8.
Lambers Heerspink HJ, Brantsma AH, de Zeeuw D, Bakker SJL, de Jong PE, Gansevoort RT. Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol. 2008;168(8):897–905.
Sarafidis PA, Riehle J, Bogojevic Z, Basta E, Chugh A, Bakris GL. A comparative evaluation of various methods for microalbuminuria screening. Am J Nephrol. 2008;28(2):324–9.
Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–80.
Ruggenenti P, Porrini E, Motterlini N, Perna A, Ilieva AP, Iliev IP, et al. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol. 2012;23(10):1717–24.
Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;128(12 Pt 1):982–8.
Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355(9200):253–9.
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9.
Jones JK, Gorkin L, Lian JF, Staffa JA, Fletcher AP. Discontinuation of and changes in treatment after start of new courses of antihypertensive drugs: a study of a United Kingdom population. BMJ. 1995;311(7000):293–5.
Caro JJ, Speckman JL, Salas M, Raggio G, Jackson JD. Effect of initial drug choice on persistence with antihypertensive therapy: the importance of actual practice data. CMAJ. 1999;160(1):41–6.
Burnier M, Hess B, Greminger P, Waeber B. Determinants of persistence in hypertensive patients treated with irbesartan: results of a postmarketing survey. BMC Cardiovasc Disord. 2005;5(1):13.
Marentette MA, Gerth WC, Billings DK, Zarnke KB. Antihypertensive persistence and drug class. Can J Cardiol. 2002;18(6):649–56.
Campbell NRC, Brant R, Johansen H, Walker RL, Wielgosz A, Onysko J, et al. Increases in antihypertensive prescriptions and reductions in cardiovascular events in Canada. Hypertension. 2009;53(2):128–34.
Acknowledgments
The in-MAU-tion study was supported by Abbott Canada. R.T. has received consulting fees from Abbott. E.B. has received lecture fees and travel support from Abbott. J.M. and A.V. are employees of Abbott. The authors are grateful to Pelle Stolt for help with medical writing and revisions to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tytus, R., Burgess, E., Maurer, J. et al. Effectiveness and Tolerability of a Trandolapril-Based Antihypertensive Treatment Regimen over 12 months in Actual Clinical Care Across Canada. Clin Drug Investig 33, 535–543 (2013). https://doi.org/10.1007/s40261-013-0092-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0092-y