Abstract
Objectives
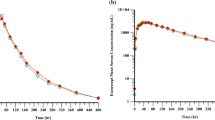
We performed this study to compare the pharmacokinetic (PK), immunogenicity, and tolerability profiles of etanercept between LBEC0101, a proposed biosimilar, and Enbrel®, the reference biological product.
Methods
A randomized, double-blind, single-dose, two-treatment, two-period, two-sequence, crossover study was conducted in 48 healthy males. In each period, a single dose of LBEC0101 or Enbrel® was subcutaneously injected at 25 mg and serial blood samples for PK evaluation were collected up to 648 h post-dose. Serum etanercept concentrations and anti-drug antibodies (ADA) were measured using an enzyme-linked immunosorbent assay and an affinity capture elution assay. Log-transformed maximum concentration (C max) and area under the concentration–time curve (AUCinf) were compared. Tolerability was also evaluated.
Results
The serum concentration–time profiles were almost overlapped between LBEC0101 and Enbrel®. Geometric mean ratio (90% confidence intervals) for C max and AUCinf of LBEC0101 to Enbrel® were 1.02 (0.92–1.13) and 0.96 (0.87–1.05), respectively, which were within a conventional bioequivalence criteria of 0.80–1.25. ADA development was also comparable. Both drugs were well tolerated.
Conclusions
LBEC0101 showed similar PK, immunogenicity, and tolerability profiles to Enbrel® after a single subcutaneous injection in healthy males. LBEC0101 can be further developed as a potential etanercept biosimilar (ClinicalTrial.gov identifier: NCT01725620).


Similar content being viewed by others
References
Goffe B, Cather JC. Etanercept: an overview. J Am Acad Dermatol. 2003;49(2 Suppl):S105–11. doi:10.1016/mjd.2003.554.
Azevedo VF, Galli N, Kleinfelder A, D’Ippolito J, Urbano PC. Etanercept biosimilars. Rheumatol Int. 2015;35(2):197–209. doi:10.1007/s00296-014-3080-5.
European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues, Committee for Medicinal Products for HUMAN Use (CHMP). London: European Medicines Agency; 2012.
Emmanouilides CE, Karampola MI, Beredima M. Biosimilars: hope and concern. J Oncol Pharm Pract. 2016;22(4):618–24. doi:10.1177/1078155215603232.
Weise M, Bielsky MC, De Smet K, Ehmann F, Ekman N, Giezen TJ, et al. Biosimilars: what clinicians should know. Blood. 2012;120(26):5111–7. doi:10.1182/blood-2012-04-425744.
Dranitsaris G, Dorward K, Hatzimichael E, Amir E. Clinical trial design in biosimilar drug development. Investig New Drugs. 2013;31(2):479–87. doi:10.1007/s10637-012-9899-2.
Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product. Rockville: Food and Drug Administration; 2012.
Scheinberg MA, Kay J. The advent of biosimilar therapies in rheumatology—”O brave new world”. Nat Rev Rheumatol. 2012;8(7):430–6. doi:10.1038/nrrheum.2012.84.
Calo-Fernandez B, Martinez-Hurtado JL. Biosimilars: company strategies to capture value from the biologics market. Pharmaceuticals (Basel). 2012;5(12):1393–408. doi:10.3390/ph5121393.
Geng D, Shankar G, Schantz A, Rajadhyaksha M, Davis H, Wagner C. Validation of immunoassays used to assess immunogenicity to therapeutic monoclonal antibodies. J Pharm Biomed Anal. 2005;39(3–4):364–75. doi:10.1016/j.jpba.2005.04.045.
Ministry of Food and Drug Safety. Guidelines on the evaluation of biosimilar products, revision 1. Chungchengbuk-do: Ministry of Food and Drug Safety; 2014.
Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Clinical pharmacology data to support a demonstration of biosimilarity to a reference product. Rockville: Food and Drug Administration; 2014.
Korth-Bradley JM, Rubin AS, Hanna RK, Simcoe DK, Lebsack ME. The pharmacokinetics of etanercept in healthy volunteers. Ann Pharmacother. 2000;34(2):161–4.
Yi S, Kim SE, Park MK, Yoon SH, Cho JY, Lim KS, et al. Comparative pharmacokinetics of HD203, a biosimilar of etanercept, with marketed etanercept (Enbrel(R)): a double-blind, single-dose, crossover study in healthy volunteers. BioDrugs. 2012;26(3):177–84. doi:10.2165/11631860-000000000-00000.
Emi Aikawa N, de Carvalho JF, Artur Almeida Silva C, Bonfa E. Immunogenicity of anti-TNF-alpha agents in autoimmune diseases. Clin Rev Allergy Immunol. 2010;38(2–3):82–9. doi:10.1007/s12016-009-8140-3.
Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25(9):1007–11. doi:10.1111/j.1468-3083.2011.04065.x.
Lee H, Kimko HC, Rogge M, Wang D, Nestorov I, Peck CC. Population pharmacokinetic and pharmacodynamic modeling of etanercept using logistic regression analysis. Clin Pharmacol Ther. 2003;73(4):348–65.
Lee YJ, Shin D, Kim Y, Kang J, Gauliard A, Fuhr R. A randomized phase l pharmacokinetic study comparing SB4 and etanercept reference product (Enbrel®) in healthy subjects. Br J Clin Pharmacol. 2016. doi:10.1111/bcp.12929.
Zhou SY, Shu C, Korth-Bradley J, Raible D, Palmisano M, Wadjula J, et al. Integrated population pharmacokinetics of etanercept in healthy subjects and in patients with rheumatoid arthritis and ankylosing spondylitis. J Clin Pharmacol. 2011;51(6):864–75. doi:10.1177/0091270010375961.
Choi Y, Jiang F, An H, Park HJ, Choi JH, Lee H. A pharmacogenomic study on the pharmacokinetics of tacrolimus in healthy subjects using the DMETTM Plus platform. Pharmacogenomics J. 2016. doi:10.1038/tpj.2015.99.
Acknowledgements
The authors thank all the staff members in the Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital and Seoul National University Hospital Clinical Trial Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The study was approved by the Institutional Review Board of Seoul National University Hospital. The study was conducted in full accordance with the ethical principles of the Declaration of Helsinki and Korean Good Clinical Practices and other applicable regulations. Informed consent was obtained from all individual participants included in the study.
Competing interests
Sung Mo Yang is an employee of LG Chem, Ltd, Republic of Korea (former LG Life Sciences, Ltd), which is the sponsor and analytical institute of this clinical study. Heechan Lee, Hyewon Chung, SeungHwan Lee, Howard Lee, Seo Hyun Yoon, Joo-Youn Cho, In-Jin Jang, and Kyung-Sang Yu have no other competing interest with regard to the content of this article.
Funding
This study was funded by LG Chem, Ltd, Republic of Korea (former LG Life Sciences, Ltd).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, H., Chung, H., Lee, S. et al. LBEC0101, A Proposed Etanercept Biosimilar: Pharmacokinetics, Immunogenicity, and Tolerability Profiles Compared with a Reference Biologic Product in Healthy Male Subjects. BioDrugs 31, 349–355 (2017). https://doi.org/10.1007/s40259-017-0230-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-017-0230-9