Abstract
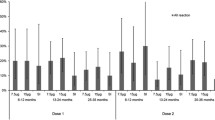
Vaccination is an essential tool in reducing the impact of seasonal influenza infections. The viral strains responsible for seasonal outbreaks vary annually, and preventive vaccines have to be adapted accordingly. The aim of this study was to evaluate the safety, clinical tolerability and the antibody response to each of the three influenza vaccine antigens after vaccination with a cell-derived, trivalent, surface antigen, inactivated influenza vaccine (TIVc), as measured by single radial haemolysis (SRH) or haemagglutination inhibition (HI) assay in accordance with European Union licensing guidelines in place for years 2013/2014. This phase 3, open-label, single-arm study enrolled 126 healthy adults divided into two age groups (63 subjects aged 18 to ≤ 60 years and 63 subjects aged ≥ 61 years). Antibody titres were measured before and 21 days after vaccination. Adverse events were determined using diary cards, interviews and reviews of the available medical records. One subject was lost to follow-up and three subjects had protocol deviations. Following vaccination, protective HI antibody titres (≥ 1:40) were detected in 100%, 97%, and 94% of the younger adults (18–≤ 60 years) and in 97%, 95%, and 80% of the older adults (≥ 61 years) against the A (H1N1), A (H3N2), and B influenza strains respectively. The antibody response licensing criteria were met in both age groups. Solicited adverse events were reported by 57% subjects 18 to ≤ 60 years and 35% subjects ≥ 61 years. Among the younger adults 51% had local and 27% had systemic adverse events, whereas of the older subjects 29% had local and 13% had systemic adverse events (mainly injection site pain or headache in both age groups). Unsolicited adverse events at least possibly related to the vaccine were mild and detected in 3% of the younger adults and none of the older adults. Overall, the trivalent, surface antigen, inactivated subunit influenza virus vaccine produced in mammalian cell culture proved to be safe and immunogenic in younger and older healthy adults.
Similar content being viewed by others
References
Puig-Barberà J, Burtseva E, Yu H, Cowling BJ, Badur S, Kyncl J, et al. Influenza epidemiology and influenza vaccine effectiveness during the 2014–2015 season: annual report from the Global Influenza Hospital Surveillance Network. BMC Public Health. 2016;16:757. https://doi.org/10.1186/s12889-016-3378-1.
Alonso WJ, Yu C, Viboud C, Richard SA, Schuck-Paim C, Simonsen L, et al. A global map of hemispheric influenza vaccine recommendations based on local patterns of viral circulation. Sci Rep. 2015;5:17214. https://doi.org/10.1038/srep17214.
Recommended composition of influenza virus vaccines for use in the 2013–14 northern hemisphere influenza season (database on the Internet). WHO. 2013. http://www.who.int/entity/influenza/vaccines/virus/recommendations/201302_recommendation.pdf?ua=1. Accessed 29 May 2016.
Doroshenko A, Halperin SA. Trivalent MDCK cell culture-derived influenza vaccine Optaflu (Novartis vaccines). Expert Rev Vaccines. 2009;8:679–88. https://doi.org/10.1586/erv.09.31.
Frey S, Vesikari T, Szymczakiewicz-Multanowska A, Lattanzi M, Izu A, Groth N, et al. Clinical efficacy of cell culture-derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis. 2010;51:997–1004. https://doi.org/10.1086/656578.
Loebermann M, Voss U, Meyer S, Bosse D, Fritzsche C, Klammt S, et al. Clinical trial to evaluate the safety and immunogenicity of a trivalent surface antigen seasonal influenza vaccine produced in mammalian cell culture and administered to young and elderly adults with and without A (H1N1) pre-vaccination. PLoS One. 2013;8:e70866. https://doi.org/10.1371/journal.pone.0070866.
Vinnemeier CD, Fischer-Herr J, Meyer S, Liebig K, Theess W, Burchard GD, et al. Immunogenicity and safety of an inactivated 2012/2013 trivalent influenza vaccine produced in mammalian cell culture (Optaflu(R)): an open label, uncontrolled study. Hum Vaccin Immunother. 2014;10:441–8. https://doi.org/10.4161/hv.27140.
Note for Guidance on Harmonisation of Requirements for Influenza Vaccines (database on the Internet). European Agency for the Evaluation of Medicinal Products (EMEA). 1997. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf. Accessed 15 Jun 2018.
Loebermann M, Anders G, Brestrich G, Fritzsche C, Klammt S, Borso D, et al. Safety and immunogenicity of a trivalent single dose seasonal influenza vaccine containing pandemic A (H1N1) antigen in younger and elderly subjects: a phase III open-label single-arm study. Vaccine. 2011;29:1228–34. https://doi.org/10.1016/j.vaccine.2010.11.092.
Felldin M, Andersson B, Studahl M, Svennerholm B, Friman V. Antibody persistence 1 year after pandemic H1N1 2009 influenza vaccination and immunogenicity of subsequent seasonal influenza vaccine among adult organ transplant patients. Transpl Int. 2014;27:197–203. https://doi.org/10.1111/tri.12237.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare the following conflicts of interest: EH and DK are employees of Seqirus, the company that acquired the sponsors’ influenza vaccine division; ML and ECR have served as principal investigator or coordinating investigator for studies sponsored by GSK, Novartis Vaccines, Seqirus, and Valneva; all other authors declare that no competing interests exist.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Loebermann, M., Fritzsche, C., Geerdes-Fenge, H. et al. A phase III, open-label, single-arm, study to evaluate the safety and immunogenicity of a trivalent, surface antigen inactivated subunit influenza virus vaccine produced in mammalian cell culture (Optaflu®) in healthy adults. Infection 47, 105–109 (2019). https://doi.org/10.1007/s15010-018-1233-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-018-1233-2