Abstract
Background
Protein arginine methyltransferase 1 (PRMT1) is the founding member of the PRMT family of proteins, whose members catalyze methylation of arginine residues in various proteins. Although several studies have reported upregulation of PRMT1 in various cancer types, the expression pattern and the underlying mechanism of PRMT1 action in pancreatic ductal adenocarcinoma (PDAC) are still unclear.
Methods
Immunohistochemistry staining as well as RT-PCR was used to determine the expression pattern of PRMT1 in clinical PDAC samples. Lentivirus packaging and transfection were employed to construct cell lines with PRMT1 overexpression or knockdown. MTT and crystal violet assays were used to determine the proliferation rates of PDAC cells. β-catenin transcription activity was measured using a TOPFlash assay. PRMT1 binding to the promoter region of CTNNB1 was determined by ChIP-qPCR assay.
Results
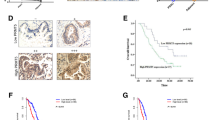
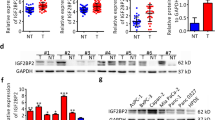
Elevated PRMT1 expression was found in PDAC tissue samples compared to noncancerous normal tissues in 41 patients using a real-time PCR assay and in 90 patients using a tissue microarray (TMA) in conjunction with immunohistochemistry. Analysis of the PRMT1 expression data and PDAC clinical features revealed that PRMT1 expression was significantly correlated with PDAC tumor size and prognosis in postoperative patients. Additional functional experiments revealed that PRMT1 expression promoted the growth of pancreatic cancer-derived cells, both in vitro and in vivo. Mechanistically, we found that PRMT1 increased the cellular β-catenin level. We also found that PRMT1 and β-catenin were co-expressed in TCGA and GTEx datasets containing 370 samples.
Conclusions
Collectively, our study provides novel insight into the expression and function of PRMT1 in PDAC and indicates that PRMT1 may serve as a therapeutic target for treating patients with pancreatic ductal adenocarcinoma.





Similar content being viewed by others
Change history
02 December 2019
The authors would like to correct the following panels in Figures
References
R.L. Siegel, K.D. Miller, A. Jemal, Cancer statistics, 2017. CA Cancer J. Clin. 67, 7–30 (2017)
R. Siegel, J.M. Ma, Z.H. Zou, A. Jemal, Cancer statistics, 2014. CA Cancer J. Clin. 64, 9–29 (2014)
T. Kamisawa, L.D. Wood, T. Itoi, K. Takaori, Pancreatic cancer. Lancet 388, 73–85 (2016)
L.B. Saltz, P.B. Bach, Albumin-bound paclitaxel plus gemcitabine in pancreatic cancer. New Engl. J. Med. 370, 478–478 (2014)
A. Makohon-Moore, C.A. Iacobuzio-Donahue, Pancreatic cancer biology and genetics from an evolutionary perspective. Nat. Rev. Cancer 16, 553–565 (2016)
A.H. Bild, A. Potti, J.R. Nevins, Linking oncogenic pathways with therapeutic opportunities. Nat. Rev. Cancer 6, 735–U713 (2006)
C. Busonero, S. Leone, F. Acconcia, Emetine induces estrogen receptor alpha degradation and prevents 17 beta-estradiol-induced breast cancer cell proliferation. Cell. Oncol. 40, 299–301 (2017)
M.S.G. Montani, M. Granato, C. Santoni, P. Del Porto, N. Merendino, G. D'Orazi, A. Faggioni, M. Cirone, Histone deacetylase inhibitors VPA and TSA induce apoptosis and autophagy in pancreatic cancer cells. Cell. Oncol. 40, 167–180 (2017)
P.A. Futreal, L. Coin, M. Marshall, T. Down, T. Hubbard, R. Wooster, N. Rahman, M.R. Stratton, A census of human cancer genes. Nat. Rev. Cancer 4, 177–183 (2004)
R. Hamamoto, V. Saloura, Y. Nakamura, Critical roles of non-histone protein lysine methylation in human tumorigenesis. Nat. Rev. Cancer 15, 110–124 (2015)
R.R. Yakubu, N.C. Silmon de Monerri, E. Nieves, K. Kim, L.M. Weiss, Comparative Monomethylarginine proteomics suggests that protein arginine methyltransferase 1 (PRMT1) is a significant contributor to arginine Monomethylation in toxoplasma gondii. Mol. Cell. Proteomics 16, 567–580 (2017)
M.H. Jun, H.H. Ryu, Y.W. Jun, T. Liu, Y. Li, C.S. Lim, Y.S. Lee, B.K. Kaang, D.J. Jang, J.A. Lee, Sequestration of PRMT1 and Nd1-L mRNA into ALS-linked FUS mutant R521C-positive aggregates contributes to neurite degeneration upon oxidative stress. Sci. Rep. 7, 40474 (2017)
Z. Fan, J. Li, P. Li, Q. Ye, H. Xu, X. Wu, Y. Xu, Protein arginine methyltransferase 1 (PRMT1) represses MHC II transcription in macrophages by methylating CIITA. Sci. Rep. 7, 40531 (2017)
R.S. Blanc, G. Vogel, X. Li, Z. Yu, S. Li, S. Richard, Arginine methylation by PRMT1 regulates muscle stem cell fate. Mol. Cell. Biol. 37, e00457–16 (2017)
H.W. Liao, J.M. Hsu, W. Xia, H.L. Wang, Y.N. Wang, W.C. Chang, S.T. Arold, C.K. Chou, P.H. Tsou, H. Yamaguchi, Y.F. Fang, H.J. Lee, H.H. Lee, S.K. Tai, M.H. Yang, M.P. Morelli, M. Sen, J.E. Ladbury, C.H. Chen, J.R. Grandis, S. Kopetz, M.C. Hung, PRMT1-mediated methylation of the EGF receptor regulates signaling and cetuximab response. J. Clin. Invest. 125, 4529–4543 (2015)
B. Cha, W. Kim, Y.K. Kim, B.N. Hwang, S.Y. Park, J.W. Yoon, W.S. Park, J.W. Cho, M.T. Bedford, E.H. Jho, Methylation by protein arginine methyltransferase 1 increases stability of Axin, a negative regulator of Wnt signaling. Oncogene 30, 2379–2389 (2011)
B. Li, L. Liu, X. Li, L. Wu, miR-503 suppresses metastasis of hepatocellular carcinoma cell by targeting PRMT1. Biochem. Biophys. Res. Commun. 464, 982–987 (2015)
B. Altan, T. Yokobori, M. Ide, E. Mochiki, Y. Toyomasu, N. Kogure, A. Kimura, K. Hara, T. Bai, P. Bao, M. Suzuki, K. Ogata, T. Asao, M. Nishiyama, T. Oyama, H. Kuwano, Nuclear PRMT1 expression is associated with poor prognosis and chemosensitivity in gastric cancer patients. Gastric Cancer 19, 789–797 (2016)
Y.Z. Deng, F. Yao, J.J. Li, Z.F. Mao, P.T. Hu, L.Y. Long, G. Li, X.D. Ji, S. Shi, D.X. Guan, Y.Y. Feng, L. Cui, D.S. Li, Y. Liu, X. Du, M.Z. Guo, L.Y. Xu, E.M. Li, H.Y. Wang, D. Xie, RACK1 suppresses gastric tumorigenesis by stabilizing the beta-catenin destruction complex. Gastroenterology 142, 812–823 e815 (2012)
E.C. Stack, C. Wang, K.A. Roman, C.C. Hoyt, Multiplexed immunohistochemistry, imaging, and quantitation: A review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 70, 46–58 (2014)
J.J. Li, D.P. Liu, D. Xie, EphrinA5 acts as a tumor suppressor in glioma by negative regulation of epidermal growth factor receptor. Cancer Res. 69, 1759–1768 (2009)
Z. Cai, Z.Y. Qian, H. Jiang, N. Ma, Z. Li, L.Y. Liu, X.X. Ren, Y.R. Shang, J.J. Wang, J.J. Li, D.P. Liu, X.P. Zhang, D. Feng, Q.Z. Ni, Y.Y. Feng, N. Li, X.Y. Zhou, X. Wang, Y. Bao, X.L. Zhang, Y.Z. Deng, D. Xie, hPCL3s promotes hepatocellular carcinoma metastasis by activating beta-catenin signaling. Cancer Res. 78, 2536–2549 (2018)
Z. Tang, C. Li, B. Kang, G. Gao, C. Li, Z. Zhang, GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45, W98–W102 (2017)
Y.Z. Deng, Z. Cai, S. Shi, H. Jiang, Y.R. Shang, N. Ma, J.J. Wang, D.X. Guan, T.W. Chen, Y.F. Rong, Z.Y. Qian, E.B. Zhang, D. Feng, Q.L. Zhou, Y.N. Du, D.P. Liu, X.X. Huang, L.M. Liu, E. Chin, D.S. Li, X.F. Wang, X.L. Zhang, D. Xie, Cilia loss sensitizes cells to transformation by activating the mevalonate pathway. J. Exp. Med. 215, 177–195 (2018)
L. Yu, X. Li, H. Li, H. Chen, H. Liu, Rab11a sustains GSK3beta/Wnt/beta-catenin signaling to enhance cancer progression in pancreatic cancer. Tumour Biol. 37, 13821–13829 (2016)
M. Ji, D. Fan, L. Yuan, Y. Zhang, W. Dong, X. Peng, EBP50 inhibits pancreatic cancer cell growth and invasion by targeting the beta-catenin/E-cadherin pathway. Exp. Ther. Med. 10, 1311–1316 (2015)
W. Zhou, Y. Li, S. Gou, J. Xiong, H. Wu, C. Wang, H. Yan, T. Liu, MiR-744 increases tumorigenicity of pancreatic cancer by activating Wnt/beta-catenin pathway. Oncotarget 6, 37557–37569 (2015)
M.T. Bedford, Arginine methylation at a glance. J. Cell Sci. 120, 4243–4246 (2007)
Y. Wang, J.M. Hsu, Y. Kang, Y. Wei, P.C. Lee, S.J. Chang, Y.H. Hsu, J.L. Hsu, H.L. Wang, W.C. Chang, C.W. Li, H.W. Liao, S.S. Chang, W. Xia, H.W. Ko, C.K. Chou, J.B. Fleming, H. Wang, R.F. Hwang, Y. Chen, J. Qin, M.C. Hung, Oncogenic functions of Gli1 in pancreatic adenocarcinoma are supported by its PRMT1-mediated methylation. Cancer Res. 76, 7049–7058 (2016)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant number: 81572294) to Wenhui Lou. We thank professor YZ. Deng for his writing assistance and Dr. H Jiang for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest statement
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 341 kb)
Rights and permissions
About this article
Cite this article
Song, C., Chen, T., He, L. et al. PRMT1 promotes pancreatic cancer growth and predicts poor prognosis. Cell Oncol. 43, 51–62 (2020). https://doi.org/10.1007/s13402-019-00435-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-019-00435-1