Abstract
Introduction
The aim of this study was to evaluate the efficacy and safety of initial combination therapy compared with monotherapy in drug-naïve type 2 diabetes patients.
Methods
MEDLINE, Embase and the Cochrane Central Register of Controlled Trials were searched for randomized clinical trials of initial combination therapy with hypoglycemic agents compared with monotherapy. Those which satisfied the search criteria were included in the meta-analysis. Weighted mean difference and relative risks were calculated.
Results
A total of 36 studies were included in the meta-analysis. Compared with metformin monotherapy, initial combination therapy with metformin plus another anti-diabetes drug exhibited significant reductions in glycated hemoglobin (HbA1c) (p < 0.001). Most of the combination therapies had a similar risk of hypoglycemia (p > 0.05), with the exception of combinations of sulfonylurea/glinide and metformin or combinations of thiazolidinedione and metformin. Compared with dipeptidyl peptidase-4 (DPP-4) inhibitor monotherapy, initial combination therapy with DPP-4 inhibitor plus another anti-diabetes drug showed a significant decrease in HbA1c (p < 0.001) and a similar risk of hypoglycemia (p > 0.05). Compared with monotherapy with other anti-diabetes drugs, initial combination therapies also resulted in significant HbA1c reductions, a similar risk of hypoglycemia and similar risks of other adverse events.
Conclusion
Compared with monotherapy, all initial combination therapies resulted in significant HbA1c reductions. Compared with metformin monotherapy, initial combination therapies with DPP-4 inhibitors plus metformin, sodium/glucose cotransporter 2 inhibitors and metformin, respectively, were associated with similar risks of hypoglycemia, but initial combination therapies with sulfonylurea plus metformin, thiazolidinedione and metformin, respectively, were associated with higher risks of hypoglycemia.
Funding
AstraZeneca Ltd. (China).
Trial registration
Registration number CRD42017060717 in PROSPERO.
Similar content being viewed by others

Introduction
Initial hypoglycemic monotherapy is usually used in newly diagnosed type 2 diabetes patients, as currently recommended by the guidelines of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [1, 2]. However, initial monotherapy is frequently insufficient to enable patients to achieve or sustain glycemic targets [3, 4]. Thus, initial combination therapy has emerged as an alternative approach. The latest position statement from the ADA/EASD [2] called for an initial combination of two non-insulin agents in patients with a high baseline glycated hemoglobin (HbA1c) level (≥ 9.0%). Additionally, the latest American Association of Clinical Endocrinologists (AACE) treatment algorithm [5] recommended that patients with a HbA1c level of > 7.5% should receive combination therapy with metformin plus an additional drug.
However, we asked the question of whether initial combination therapy is actually more efficacious than monotherapy in terms of glucose control and confirmed safety. To search for the answer, we identified two published systematic reviews and meta-analyses. In one meta-analysis [6] that included 15 randomized controlled trials (RCTs), the authors found that compared to metformin alone, combination therapy with metformin plus another anti-diabetes drug provided statistically significant reductions of 0.43% in HbA1c level and of 14.30 mg/dl in fasting plasma glucose (FPG) level. In another meta-analysis [7] that included eight RCTs, the authors reported that compared with metformin monotherapy, initial combination therapy with dipeptidyl peptidase-4 (DPP-4) inhibitors plus metformin was associated with a higher reduction of 0.49% in HbA1c level, a higher reduction of 0.80 mmol/l in FPG level and a lower weight loss of 0.44 kg. However, the authors of both of these meta-analyses did not present any further analysis with regard to the different types of hypoglycemic agent tested. Therefore, the aims of this study reported here were to comprehensively evaluate the efficacy and safety of initial combination therapies versus monotherapy using updated trial data in type 2 diabetes patients.
Methods
Literature Search
According to recommendations from the Cochrane Handbook for Systematic Reviews for meta-analysis, two independent investigators (XYG and WJY) conducted systematic searches of MEDLINE, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL) for studies published between the date of inception and April 2017. The search terms were: “type 2 diabetes,” “initial combination therapy,” “early combination therapy,” “treatment-naïve,” “drug-naïve,” “newly diagnosed diabetes” and “randomized controlled trials.” Treatment-naïve or drug-naïve patients were defined as those patients diagnosed with type 2 diabetes who have not received treatment with any hypoglycemic agent. “Newly diagnosed diabetes patients” were defined as those patients diagnosed with type 2 diabetes for the first time and who had not received treatment. “Early combination studies” referred to the initial combination therapy for type 2 diabetes patients. This meta-analysis is registered as CRD42017060717 in PROSPERO (International Prospective Register of Systematic Reviews).
Study Selection and Data Extraction
The inclusion criteria for this meta-analysis were: (1) studies of initial combination therapy with hypoglycemic agents compared with monotherapy; (2) efficacy of glucose control was the primary outcome of the study; (3) double-blind RCTs; (4) studies conducted with treatment-naïve type 2 diabetes patients. The exclusion criteria were: (1) studies conducted in type 1 diabetes patients; (2) the study was an extension study and not the original one; (3) study duration of < 12 weeks.
Using the above inclusion and exclusion criteria, XYG and WJY independently evaluated the eligibility of all the studies identified in their search MEDLINE, Embase and CENTRAL. The Cochrane Collaboration tool [8] was used to rate each RCT as having a low, high or unclear risk of bias from the following aspects: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, as well as other sources of bias (Electronic Supplementary Material [ESM] Table S1 and Fig. S1). WJY and XYG then extracted details from each article, including the publication data, study design, baseline characteristics, treatment arms, study duration, changes in glucose and weight control and the hypoglycemic rate. If several doses were used in one trial, the standard doses recommended and approved in the clinical practice were documented (ESM Table S2). The definition of drug-naïve patients and the percentage of drug-naïve patients in each treatment arm were also documented (ESM Table S3).
Statistical Analyses
The primary endpoint of this meta-analysis was the change in HbA1c level from baseline to the study endpoint in patients who received initial combination therapies compared with those receiving monotherapy. The secondary endpoints included changes in FPG, postprandial glucose (PPG) and body weight and the risk of hypoglycemia in patients who received initial combination therapies compared with those receiving monotherapy. Continuous outcomes were evaluated by computing the weighted mean differences (WMDs) and the 95% confidence intervals (CIs). Categorical outcomes were evaluated by computing the relative risks (RRs) and accompanying 95% CIs. Due to between-study heterogeneity, Higgins I2 statistics were used to evaluate the percentage of variance. Heterogeneity can be quantified as low, moderate and high, with upper limits of 25, 50 and 75% for I2, respectively [9,10,11]. The 95% CIs of I2 were also calculated [11]. Publication bias was assessed using a funnel plot (ESM Fig. S2).
Meta-regression analysis was performed to evaluate whether the pre-specified covariates of baseline age, gender, HbA1c level and baseline body mass index (BMI) were associated with HbA1c changes from baseline corrected by monotherapy. Differences were considered to be statistically significant as p < 0.05.
Statistical analyses were primarily performed using the Review Manager statistical software package (version 5.2; Nordic Cochrane Centre, Copenhagen, Denmark). Analyses were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for conducting and reporting meta-analyses of RCTs [12]. Meta-regression analyses were performed using the STATA statistical software package (version 11.0; StataCorp, College Station, TX, USA).
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Characteristics and Methodological Quality of Included Studies
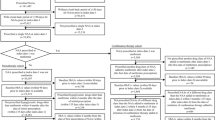
A total of 36 studies were included in the meta-analysis (Fig. 1; Table 1). Of these, 12 were studies [13,14,15,16,17,18,19,20,21,22,23,24] with initial combination therapies of DPP-4 inhibitors plus metformin, three were studies [25,26,27] in which the initial combination therapy was sulfonylurea (SU) or glinide plus metformin, four were studies [28,29,30,31] in which the initial combination therapy was thiazolidinedione (TZD) plus metformin, three were studies [32,33,34] in which the initial combination therapy was sodium glucose cotransporter 2 (SGLT2) inhibitor plus metformin and six studies [35,36,37,38,39,40] utilized an initial combination therapy of DPP-4 inhibitor plus TZD. There were also eight trials with other initial combination therapies [41,42,43,44,45,46,47,48].
Our meta-analysis included studies that were randomized, placebo-controlled, with double-blind treatment. The eligibility criteria were clearly reported in all of the trials. Most studies reported baseline age, BMI, HbA1c level and duration of diabetes between the comparison groups. The risk of bias as evaluated by the Cochrane instrument was low (ESM Fig. S1). The visual inspection of the funnel plots indicated low risks of publication bias (ESM Fig. S2). For some treatment groups included only one trial, no further meta-analysis was done in each group [41,42,43,44,45,46,47,48]. Those extension studies were excluded from this meta-analysis.
Efficacy of Initial Combination Therapy
Compared with metformin monotherapy, initial combinations of DPP-4 inhibitors and metformin exhibited significant decreases in HbA1c (WMD, − 0.44%, p < 0.001), FPG (WMD, − 0.77 mmol/l, p < 0.001) and PPG (WMD, − 1.65 mmol/l, p < 0.001), but increased body weight significantly (WMD, 0.38 kg, p < 0.001). Compared with DPP-4 inhibitors monotherapy, initial combinations of DPP-4 inhibitors and metformin caused significant decreases in HbA1c (WMD, − 0.88%, p < 0.001), FPG (WMD, − 1.61 mmol/l, p < 0.001), PPG (WMD, − 2.69 mmol/l, p < 0.001) and body weight (WMD, − 1.00 kg, p < 0.001) (Table 2; Figure S3).
Compared with metformin monotherapy, initial treatment combinations of SU/glinides plus metformin resulted in significant decreases in the levels of HbA1c (WMD − 0.68%; p < 0.001), FPG (WMD,− 0.87 mmol/l; p < 0.001) and PPG (WMD − 0.70 mmol/l; p < 0.001), but significant increases in body weight (WMD 2.60 kg; p < 0.001). Compared with SU/glinide monotherapy, initial combinations of SU/glinides plus metformin exhibited significant decreases in the levels of HbA1c (WMD − 0.49%; p < 0.001), FPG (WMD − 0.66 mmol/l; p = 0.005) and PPG (WMD − 0.87 mmol/l; p < 0.001) and similar changes in weight (WMD − 0.10 kg; p = 0.74) (Table 2; ESM Fig. S3).
Compared with metformin monotherapy, initial combinations of TZDs plus metformin led to significant decreases in HbA1c (WMD − 0.44%; p < 0.001) and FPG levels (WMD, − 0.88 mmol/l; p < 0.001) but increased body weight significantly (WMD 1.93 kg; p < 0.001). Compared with TZD monotherapy, initial combinations of TZDs plus metformin led to significant decreases in the levels of HbA1c (WMD − 0.83%; p < 0.001) and FPG (WMD − 1.25 mmol/l; p < 0.001) and body weight (WMD − 1.22 kg; p < 0.001) (Table 2; ESM Fig. S3).
Initial combinations of SGLT2 inhibitors plus metformin led to significant decreases in HbA1c (WMD, − 0.47%, p < 0.001), FPG (WMD, − 1.38 mmol/l, p < 0.001) and body weight (WMD, − 2.00 kg, p < 0.001) when compared with metformin monotherapy. Initial combinations of SGLT2 inhibitors plus metformin also led to significant decreases in HbA1c (WMD − 0.64%; p < 0.001) and FPG (WMD − 0.83 mmol/l; p < 0.001) levels and body weight (WMD − 0.66 kg; p < 0.001) when compared to SGLT2 inhibitor monotherapy (Table 2; ESM Fig. S3).
Compared with TZD monotherapy, initial combinations of DPP-4 inhibitors plus TZD exhibited significant decreases in the levels of HbA1c (WMD − 0.54%; p < 0.001), FPG (WMD − 0.89 mmol/l; p < 0.001) and PPG (WMD − 1.97 mmol/l; p < 0.001) but increased body weight significantly (WMD 0.96 kg; p < 0.001). Compared with DPP-4 inhibitor monotherapy, initial combinations of DPP-4 inhibitors plus TZD resulted in significant decreases in HbA1c (WMD − 0.62%; p < 0.001) and FPG (WMD − 1.41 mmol/l; p < 0.001) levels but significant increases in body weight (WMD 3.51 kg; p < 0.001) (Table 2; ESM Fig. S3).
Meta-regression analysis indicated that compared with monotherapy, the decrease in HbA1c level from baseline at initial combination therapy in each treatment group was not associated with the baseline HbA1c level adjusted by age, gender, and baseline BMI. However, when all data were pooled together, adjusted by age, gender and baseline BMI, HbA1c changes from baseline in the total combination therapy corrected by monotherapy was associated with baseline HbA1c level (coefficient − 2.98, 95% CI − 5.32 to − 0.63; p = 0.014) (ESM Table S4).
Adverse Effects of Initial Combination Therapy
Compared with metformin monotherapy, initial combinations of DPP-4 inhibitors plus metformin did not increase the risks of hypoglycemia, serious adverse effects (SAEs) or gastrointestinal (GI) side effects or the risk of discontinuation due to adverse effects (AEs) or drug-related AEs. When compared with DPP-4 inhibitor monotherapy, initial combinations of DPP-4 inhibitors plus metformin significantly increased the risks of hypoglycemia (RR 1.84; p = 0.007) and GI side effects (RR 2.19; p < 0.001) and the risk of drug-related AEs (RR, 1.73, p < 0.001).
Compared with metformin monotherapy, initial combinations of SU/glinides plus metformin significantly increased the risk of hypoglycemia (RR 8.91; p = 0.02). Compared with SU/glinide monotherapy, initial combinations of SU/glinides plus metformin significantly decreased the risk of hypoglycemia (RR 0.63; p < 0.001) but increased the risk of GI side effects (RR 1.42; p = 0.01).
Compared with metformin monotherapy, initial combinations of TZDs and metformin significantly increased the risk of hypoglycemia (RR 1.60; p = 0.03). Compared with TZD monotherapy, initial combinations of TZDs plus metformin did not increase the risks of any AEs.
Compared with metformin monotherapy, initial combinations of SGLT2 inhibitors and metformin significantly increased the risk of drug-related AEs (RR 1.45; p = 0.004). Compared with SGLT2 inhibitor monotherapy, initial combinations of SGLT2 inhibitors plus metformin significantly increased the risks of hypoglycemia (RR 2.23; p = 0.02) and GI side effects (RR 1.99; p = 0.002).
Compared with DPP-4 inhibitor monotherapy or TZD monotherapy, initial combinations of DPP-4 inhibitors plus TZD did not increase any risk of AEs (Table 3).
Subgroup Analysis and Sensitivity Analysis
The data were further analyzed by stratification by the study time periods. Since most studies were conducted with a 24-week follow-up, therefore, subgroup analyses were made in those studies which reported on a 24-week period of outcomes. These studies showed similar comparison results between initial combination therapy and monotherapy (ESM Table S5). We also included and excluded the study with the longest study duration of 80 weeks [31] for sensitivity analysis and found the results were all similar with the total ones. Moreover, there were several studies including both drug-naïve patients and patients previously on anti-hyperglycemia agents [13, 17, 20, 27, 29, 39, 40], in which the percentage of drug-naïve patients ranged from 50 to 90% (ESM Table S3). We also conducted a sensitivity analysis and found similar results as those for the efficacy and safety evaluations.
Discussion
Montherapy is unlike to achieve glycemic targets in patients with a high baseline HbA1c level (≥ 9%) [2], and in such cases the guidelines of the ADA/EASD recommend that the patient receive initial combination therapy [2]. In terms of “high” baseline HbA1c level, the AACE recommends initial pharmacologic combination treatment in patients with a HbA1c level of > 7.5% [5], and the Canadian Diabetes Association recommends initial combination therapy in patients with a HbA1c level of > 8.5% [49]. Among all sets of guidelines, the justification for initiating combination therapy is that patient would be unlikely to reach the glycemic target with monotherapy. The results of our meta-analysis supports that rationale, with most initial combination therapies—compared with monotherapy—showing superior glucose control in type 2 diabetes patients with an initial HbA1c level of > 7.5% at a similar risk of hypoglycemia.
As previously indicated [50, 51], there are a number of rationales for initial combination therapy in patients with type 2 diabetes. First, such therapy may lead to early robust lowering of HbA1c levels; as demonstrated by our meta-analysis, most initial combination therapies showed superior glucose control compared to monotherapy. Second, initial combination therapy may avoid the clinical inertia associated with a stepwise approach to therapy. The authors of one study suggested that the time to receive additional anti-hyperglycemic medication exceeded 1 year for patients who failed metformin monotherapy and that this delay was associated with clinical inertia [52]. Consequently, initial combination therapy may one of the best options to directly address the causes of clinical inertia [52]. Third, initial combination therapy may improve ß-cell function [50, 51]. However, this finding was not clearly evident in our meta-analysis due to the lack of data. Fourth, the complementary mechanisms of action provided by initial combination therapy may require comparatively lower doses of individual agents and therefore may cause fewer AEs. This benefit was indicated by the results of our meta-analysis which showed that most initial combination therapies exhibited better glucose control with comparable risks of hypoglycemia, SAEs, discontinuation due to AEs and GI side effects. Fifth, initial combination therapy may avoid the long-term consequences of metabolic memory, as the initial use of combination therapy could lead to greater HbA1c reduction, enabling more individuals to achieve their glycemic goals while avoiding AEs stemming from multiple metabolic defects [51, 53, 54]. However, this latter potential benefit may not be concluded from the present meta-analysis because most of the studies included were of short-term duration.
The evidence is compelling that type 2 diabetes is a progressive, physiologically and genetically complex heterogeneous disease. Achieving glycemic control is necessary to prevent or delay the progression of vascular complications. As current treatment approaches do not adequately acknowledge the complexity of diabetes, a compelling case may be made for combination treatment [51]. Initial combination therapy may be required to address the complex pathophysiology of type 2 diabetes, which includes improving insulin secretion and insulin sensitivity, inhibiting hepatic glucose production and addressing delayed gastric emptying or glucose absorption, while focusing on satiety and renal glucosuria. Among the mechanisms of hypoglycemic agents [55], metformin inhibits hepatic gluconeogenesis and improves peripheral insulin sensitivity, SUs/glinides stimulate insulin secretion by β-cells, DPP-4 inhibitors stimulate insulin secretion and suppress glucagon secretion, SGLT2 inhibitors reduce renal glucose reabsorption and induce urinary glucose excretion, TZDs activate peroxisome proliferator-activated receptor gamma (PPAR-γ) and increase insulin sensitivity. Therefore, choices for initial combinations of the above agents should also be supported by the pathophysiology of type 2 diabetes.
However, a number of unresolved issues associated with initial combination therapy in type 2 diabetes patients remain. One of these is whether initial combination therapy improve adherence. To date, there is no evidence suggesting that initial combination therapy versus monotherapy or sequential titration therapy would result in a greater adherence of patients to the therapeutic regimen. However, published studies do show that the more complex the drug regimen, the lower the adherence to that regimen [56]. In our meta-analysis, we did not collect any data on a possible improvement in adherence. Another issue is cost; is initial combination therapy less costly? The relatively high cost of including novel agents, such as DPP-4 inhibitors or SGLT2 inhibitors, in an initial combination with metformin remains a significant barrier to their use in many regions of the world [51]. Several studies have estimated the cost-effectiveness associated with monotherapy compared to combination therapy with oral anti-diabetes agents, but a number of these these were derived from non-RCT data and had multiple confounders [57, 58]. Moreover, the authors of another study indicated that it was difficult to quantify the cost-effectiveness of softer outcomes such as fewer hypoglycemic events or improved quality of life [59]. We did not collect any data on the costs of initial combination therapy in our meta-analysis, but there are other economic models which could be used to answer this question. Moreover, the association between initial combination therapy and cardiovascular risk has not been fully examined in the literature. Gaps still exist in the evidence on treatment paradigms utilizing sequential versus initial combination therapy. Therefore, carefully designed, pragmatic, prospective real-world studies to assess the clinical effectiveness of initial combinations versus sequential treatment in patients with newly diagnosed or poorly controlled type 2 diabetes should be performed to provide more evidence.
There were several limitations to our meta-analysis. First, data from the separate studies covered different durations of the study. As previously indicated, RRs are sensitive to the length of the follow-up; consequently, the pooling of results from studies with different durations of follow-up might lead to an artificial heterogeneity and discrepancy in the meta-analyses [60]. We therefore explored the outcomes in subgroup analyses by pooling all of the studies with a study period of 24 weeks to conduct a sensitivity analysis, which showed similar results with the total results. Second, the definitions of treatment-naïve patients varied depending on the protocols of the trials included in our meta-analysis, and these differences may also be associated with the high heterogeneity of this study and also lower the ability of the authors of this study to propose solid conclusions. Therefore, we also conducted a sensitivity analysis to minimize the bias and found the similar results to the efficacy and safety evaluations. The large differences in the number of studies for several combinations is another limitation. For those treatment groups with only one trial included [41,42,43,44,45,46,47,48], no further meta-analysis was done for evaluation purposes. Another problem may be the variations in dosages used in the different studies. Therefore, the standard doses recommended and approved in the clinical practice were used in this meta-analysis to minimize the bias. Since baseline characteristics were variable across studies, we used the random-effects model for analysis when the level of heterogeneity was high. Given these factors, we suggest that our results be interpreted cautiously.
Conclusions
In conclusion, compared with monotherapy, all initial combination therapies resulted in significantly reduced HbA1c levels in treatment-naïve type 2 diabetes patients. Compared with metformin monotherapy, the initial combination therapies of DPP-4 inhibitors plus metformin and SGLT2 inhibitors plus metformin exhibited similar risks of hypoglycemia, but the initial combination therapies of SU plus metformin and TZD plus metformin exhibited higher risks of hypoglycemia.
References
American Diabetes Association. Standards of medical care in diabetes—2017: pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40(Suppl 1):S64–74.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–79.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429–42.
Turner RC, Cull CA, Frighi V. Holman RR; UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281:2005–12.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 executive summary. Endocr Pract. 2017;23(2):207–38.
Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:410–7.
Wu D, Li L, Liu C. Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Obes Metab. 2014;16:30–7.
Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Higgins JP, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Higgins JP, Thompson S, Deeks J, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Thorlund K, Imberger G, Johnston BC, et al. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS One. 2012;7(7):e39471.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
Goldstein BJ, Feinglos MN, Lunceford JK, et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–87.
Bosi E, Dotta F, Jia Y, Goodman M. Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2009;11:506–15.
Jadzinsky M, Pfützner A, Paz-Pacheco E, et al. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab. 2009;11:611–22.
Reasner C, Olansky L, Seck TL, et al. The effect of initial therapy with the fixed-dose combination of sitagliptin and metformin compared with metformin monotherapy in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2011;13:644–52.
Haak T, Meinicke T, Jones R, et al. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2012;14:565–74.
Pratley RE, Fleck P, Wilson C. Efficacy and safety of initial combination therapy with alogliptin plus metformin versus either as monotherapy in drug-naïve patients with type 2 diabetes: a randomized, double-blind, 6-month study. Diabetes Obes Metab. 2014;16:613–21.
Ji L, Zinman B, Patel S, et al. Efficacy and safety of linagliptin co-administered with low-dose metformin once daily versus high-dose metformin twice daily in treatment-naïve patients with type 2 diabetes: a double-blind randomized trial. Adv Ther. 2015;32:201–15.
Ji L, Han P, Wang X, et al. Randomized clinical trial of the safety and efficacy of sitagliptin and metformin co-administered to Chinese patients with type 2 diabetes mellitus. J Diabetes Investig. 2016;7:727–36.
Mu Y, Pan C, Fan B, et al. Efficacy and safety of linagliptin/metformin single-pill combination as initial therapy in drug-naïve Asian patients with type 2 diabetes. Diabetes Res Clin Pract. 2017;124:48–56.
Dou J, Ma J, Liu J, et al. Efficacy and safety of saxagliptin in combination with metformin as initial therapy in Chinese patients with type 2 diabetes. Diabetes Obes Metab. 2018;20(3):590–8.
Ji L, Li L, Kuang J, et al. Efficacy and safety of fixed-dose combination therapy, alogliptin plus metformin, in Asian patients with type 2 diabetes: a phase 3 trial. Diabetes Obes Metab. 2017;19:754–8.
Ross SA, Caballero AE, Del Prato S, et al. Initial combination of linagliptin and metformin compared with linagliptin monotherapy in patients with newly diagnosed type 2 diabetes and marked hyperglycaemia: a randomized, double-blind, active-controlled, parallel group, multinational clinical trial. Diabetes Obes Metab. 2015;17(2):136–44.
Garber AJ, Larsen J, Schneider SH, et al. Simultaneous glyburide/metformin therapy is superior to component monotherapy as an initial pharmacological treatment for type 2 diabetes. Diabetes Obes Metab. 2002;4:201–8.
Garber AJ, Donovan DS, Dandona P, et al. Efficacy of glyburide/metformin tablets compared with initial monotherapy in type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3598–604.
Horton ES, Clinkingbeard C, Gatlin M, et al. Nateglinide alone and in combination with metformin improves glycemic control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care. 2000;23:1660–5.
Rosenstock J, Rood J, Cobitz A, et al. Initial treatment with rosiglitazone/metformin fixed-dose combination therapy compared with monotherapy with either rosiglitazone or metformin in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2006;8:650–60.
Stewart MW, Cirkel DT, Furuseth K, et al. Effect of metformin plus rosiglitazone compared with metformin alone on glycaemic control in well-controlled Type 2 diabetes. Diabet Med. 2006;23:1069–78.
Perez A, Zhao Z, Jacks R, Spanheimer R. Efficacy and safety of pioglitazone/metformin fixed-dose combination therapy compared with pioglitazone and metformin monotherapy in treating patients with T2DM. Curr Med Res Opin. 2009;25:2915–23.
Borges JL, Bilezikian JP, Jones-Leone AR, et al. A randomized, parallel group, double-blind, multicentre study comparing the efficacy and safety of Avandamet (rosiglitazone/metformin) and metformin on long-term glycaemic control and bone mineral density after 80 weeks of treatment in drug-naïve type 2 diabetes mellitus patients. Diabetes Obes Metab. 2011;13(11):1036–46.
Henry RR, Murray AV, Marmolejo MH, et al. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012;66:446–56.
Hadjadj S, Rosenstock J, Meinicke T, et al. Initial combination of empagliflozin and metformin in patients with type 2 diabetes. Diabetes Care. 2016;39(10):1718–28.
Rosenstock J, Chuck L, González-Ortiz M, et al. Initial combination therapy with canagliflozin plus metformin versus each component as monotherapy for drug-Naïve type 2 diabetes. Diabetes Care. 2016;39:353–62.
Rosenstock J, Kim SW, Baron MA, et al. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:175–85.
Rosenstock J, Inzucchi SE, Seufert J, et al. Initial combination therapy with alogliptin and pioglitazone in drug-naïve patients with type 2 diabetes. Diabetes Care. 2010;33:2406–8.
Yoon KH, Shockey GR, Teng R, et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and pioglitazone on glycemic control and measures of β-cell function in patients with type 2 diabetes. Int J Clin Pract. 2011;65:154–64.
Yoon KH, Steinberg H, Teng R, et al. Efficacy and safety of initial combination therapy with sitagliptin and pioglitazone in patients with type 2 diabetes: a 54-week study. Diabetes Obes Metab. 2012;14:745–52.
Gomis R, Espadero RM, Jones R, et al. Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13:653–61.
Henry RR, Staels B, Fonseca VA, et al. Efficacy and safety of initial combination treatment with sitagliptin and pioglitazone–a factorial study. Diabetes Obes Metab. 2014;16:223–30.
Tatsumi F, Hashiramoto M, Hirukawa H, et al. Concomitant use of miglitol and mitiglinide as initial combination therapy in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2013;101(1):35–44.
Chou HS, Palmer JP, Jones AR, et al. Initial treatment with fixed-dose combination rosiglitazone/glimepiride in patients with previously untreated type 2 diabetes. Diabetes Obes Metab. 2008;10(8):626–37.
Wainstein J, Katz L, Engel SS, et al. Initial therapy with the fixed-dose combination of sitagliptin and metformin results in greater improvement in glycaemic control compared with pioglitazone monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2012;14(5):409–18.
Amblee A, Lious D, Fogelfeld L. Combination of saxagliptin and metformin is effective as initial therapy in new-onset type 2 diabetes mellitus with severe hyperglycemia. J Clin Endocrinol Metab. 2016;101(6):2528–35.
Rosenstock J, Fonseca VA, Garvey WT, et al. Initial combination therapy with metformin and colesevelam for achievement of glycemic and lipid goals in early type 2 diabetes. Endocr Pract. 2010;16(4):629–40.
Mikada A, Narita T, Yokoyama H, et al. Effects of miglitol, sitagliptin, and initial combination therapy with both on plasma incretin responses to a mixed meal and visceral fat in over-weight Japanese patients with type 2 diabetes. “the MASTER randomized, controlled trial”. Diabetes Res Clin Pract. 2014;106(3):538–47.
Lewin A, DeFronzo RA, Patel S, et al. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care. 2015;38(3):394–402.
Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab. 2015;17(3):268–75.
Canadian Diabetes Association Clinical Practice Guidelines Expert Committee, Cheng AY. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can J Diabetes. 2013;37(Suppl 1):S1–3.
Liu Y, Hong T. Combination therapy of dipeptidyl peptidase-4 inhibitors and metformin in type 2 diabetes: rationale and evidence. Diabetes Obes Metab. 2014;16:111–7.
Cahn A, Cefalu WT. Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care. 2016;39[Suppl 2]:S137–45.
Fu AZ, Qiu Y, Davies MJ, et al. Treatment intensification in patients with type 2 diabetes who failed metformin monotherapy. Diabetes Obes Metab. 2011;13:765–9.
Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
Harris SB. The power of two: an update on fixed-dose combinations for type 2 diabetes. Expert Rev Clin Pharmacol. 2016;9:1453–62.
García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–94.
Ward A, O’Brien JA, Salas M. Cost-effectiveness of oral hypoglycaemic agents for the treatment of type 2 diabetes mellitus. Expert Opin Pharmacother. 2005;6(4):601–8.
Salas M, Ward A, Caro J. Health and economic effects of adding nateglinide to metformin to achieve dual control of glycosylated hemoglobin and postprandial glucose levels in a model of type 2 diabetes mellitus. Clin Ther 2002; 24(10):1690–1705.
Khazrai YM, Buzzetti R, Del Prato S, Cahn A, Raz I, Pozzilli P. The addition of E (Empowerment and Economics) to the ABCD algorithm in diabetes care. J Diabetes Complications. 2015;29:599–606.
Combescure C, Courvoisier DS, Haller G, Perneger TV. Meta-analysis of binary outcomes from two-by-two tables when the length of follow-up varies and hazards are proportional. Stat Methods Med Res. 2011;20(5):531–40.
Acknowledgements
Funding
This meta-analysis was financially supported by AstraZeneca Ltd. (China) and partially supported by the National Key R&D Program of China (2016YFC1304901). The funding agencies played no role in the study design, data collection or analysis, decision to publish or preparation of the manuscript. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. The article processing charges were funded by the authors.
Medical Writing, Editorial, and Other Assistance
The authors express their gratitude to X. Zhang, M. Wang and Z. Ye at AstraZeneca Ltd. China for assisting in the literature search during the preparation of this article. This part of work was supported by AstraZeneca Ltd. (China).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Linong Ji and Xiaoling Cai designed the manuscript. Xiaoling Cai, Xueying Gao, Wenjia Yang and Xueyao Han were responsible for the study selection and data extraction of the meta-analysis. Xiaoling Cai and Xueyao Han were responsible for the statistical analyses. Linong Ji and Xiaoling Cai were responsible for the manuscript writing.
Disclosures
Linong Ji has received fees for lecture presentations from AstraZeneca, Merck, Novartis, Lilly, Roche, Sanofi-Aventis and Takeda, consulting fees from companies including AstraZeneca, Merck, Novartis, Lilly, Roche, Sanofi-Aventis and Takeda and grants/research support from AstraZeneca, Bristol-Myers Squibb, Merck, Novartis and Sanofi-Aventis. Xiaoling Cai, Xueying Gao, Wenjia Yang and Xueyao Han have nothing to disclose.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as this study was based on published trials which were all included in the supplementary files and no datasets were generated during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6965681.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Cai, X., Gao, X., Yang, W. et al. Efficacy and Safety of Initial Combination Therapy in Treatment-Naïve Type 2 Diabetes Patients: A Systematic Review and Meta-analysis. Diabetes Ther 9, 1995–2014 (2018). https://doi.org/10.1007/s13300-018-0493-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-018-0493-2