Abstract
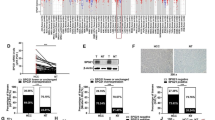
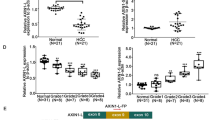
The mammalian homolog of yeast Sir2 protein is the sirtuin family of histone deacetylases (HDACs), a NAD+-dependent protein deacetylase in humans. Accumulating evidence suggests that sirtuin 2 (SIRT2) co-localizes with the microtubule network and deacetylates α-tubulin, and is involved in various cellular processes including calorie restriction-dependent life span extension, mitotic cell cycle regulation, cellular apoptosis, DNA damage repair, and genomic silencing. However, the underlying mechanisms of action remains poorly understood, especially in hepatocarcinogenesis. Hence in this study, to determine the association between the aberrant expression of SIRT2 and liver cancer development and progression, SIRT2 expression was investigated in ten selected hepatocellular carcinoma (HCC) tissues and matched normal liver tissues, using RT-PCR and Western blot analysis. Next, SIRT2 was disrupted by siRNA-mediated protein knockdown method to investigate the biological role of SIRT2 in hepatocarcinogenesis in Hep3B cells. As a result, we identified that SIRT2 expression was significantly up-regulated in HCC tissues compared to corresponding normal liver tissues. In addition, suppression of SIRT2 caused regression of tumor cell growth and proliferation. We also found that SIRT2 could interact with α-tubulin and regulates the acetylation status of α-tubulin in Hep3B cells. In conclusion, we suggest that SIRT2 is aberrantly regulated in HCCs which may contribute to the mitogenic potential of tumor cells during the development and progression of HCC, and could be a novel molecular target for therapeutic intervention in liver cancer.
Similar content being viewed by others
References
Bruix, J., Boix, L., Sala, M. & Llovet, J. M. Focus on hepatocellular carcinoma. Cancer Cell 5:215–219 (2004).
Olsen, S. K., Brown, R. S. & Siegel, A. B. Hepatocellular carcinoma: review of current treatment with a focus on targeted molecular therapies. Therap Adv Gastroenterol 3:55–66 (2010).
Bosch, F. X., Ribes, J., Cleries, R. & Diaz, M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis 9:191–211 (2005).
Yang, J. D. & Roberts, L. R. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol 7:448–458 (2010).
Kuo, M. H. & Allis, C. D. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 20:615–626 (1998).
Sengupta, N. & Seto, E. Regulation of histone deacetylase activities. J Cell Biochem 93:57–67 (2004).
Glozak, M. A. & Seto, E. Histone deacetylases and cancer. Oncogene 26:5420–5432 (2007).
Witt, O., Deubzer, H. E., Milde, T. & Oehme, I. HDAC family: What are the cancer relevant targets? Cancer Lett 277:8–21 (2009).
Frye, R. A. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 273:793–798 (2000).
Blander, G. & Guarente, L. The Sir2 family of protein deacetylases. Annu Rev Biochem 73:417–435 (2004).
Longo, V. D. & Kennedy, B. K. Sirtuins in aging and age-related disease. Cell 126:257–268 (2006).
Kennedy, B. K., Smith, E. D. & Kaeberlein, M. The enigmatic role of Sir2 in aging. Cell 123:548–550 (2005).
Gorospe, M. & de Cabo, R. AsSIRTing the DNA damage response. Trends Cell Biol 18:77–83 (2008).
Haigis, M. C. & Guarente, L. P. Mammalian sirtuinsemerging roles in physiology, aging, and calorie restriction. Genes Dev 20:2913–2921 (2006).
Michan, S. & Sinclair, D. Sirtuins in mammals: insights into their biological function. Biochem J 404:1–13 (2007).
North, B. J. et al. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11:437–444 (2003).
Hubbert, C. et al. HDAC6 is a microtubule-associated deacetylase. Nature 417:455–458 (2002).
Zhang, Y. et al. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J 22: 1168–1179 (2003).
Honore, S., Pasquier, E. & Braguer, D. Understanding microtubule dynamics for improved cancer therapy. Cell Mol Life Sci 62:3039–3056 (2005).
Dryden, S. C. et al. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol 23:3173–3185 (2003).
Pandithage, R. et al. The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell Biol 180:915–929 (2008).
Vaquero, A. et al. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev 20:1256–1261 (2006).
Black, J. C. et al. The SIRT2 deacetylase regulates autoacetylation of p300. Mol Cell 32:449–455 (2008).
Heltweg, B. et al. Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res 66:4368–4377 (2006).
Inoue, T., Hiratsuka, M., Osaki, M. & Oshimura, M. The molecular biology of mammalian SIRT proteins: SIRT2 in cell cycle regulation. Cell Cycle 6:1011–1018 (2007).
Milne, J. C. & Denu, J. M. The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol 12:11–17 (2008).
Zhang, Y. et al. Identification of a small molecule SIRT2 inhibitor with selective tumor cytotoxicity. Biochem Biophys Res Commun 386:729–733 (2009).
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403:795–800 (2000).
Lavu, S., Boss, O., Elliott, P. J. & Lambert, P. D. Sirtuins-novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov 7:841–853 (2008).
Taylor, D. M., Maxwell, M. M., Luthi-Carter, R. & Kazantsev, A. G. Biological and potential therapeutic roles of sirtuin deacetylases. Cell Mol Life Sci 65: 4000–4018 (2008).
Schemies, J., Sippl, W. & Jung, M. Histone deacetylase inhibitors that target tubulin. Cancer Lett 280:222–232 (2009).
Saunders, L. R. & Verdin, E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene 26:5489–5504 (2007).
Peck, B. et al. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther 9:844–855 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work
Rights and permissions
About this article
Cite this article
Xie, H.J., Jung, K.H. & Nam, S.W. Overexpression of SIRT2 contributes tumor cell growth in hepatocellular carcinomas. Mol. Cell. Toxicol. 7, 367–374 (2011). https://doi.org/10.1007/s13273-011-0046-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-011-0046-5