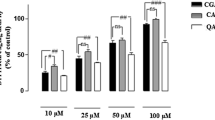
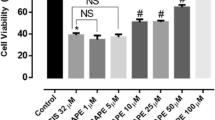
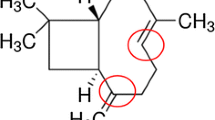
Abstract
In the CNS, including the optic nerve, oligodendrocytes play a critical role in the myelination of axons. Oligodendrocytes are exceptionally sensitive to insults to the CNS, such as injury, ischemia, or inflammation, which result in the loss of oligodendrocytes and myelin and eventually secondary axon degeneration. Oligodendrocytes are sensitive to excitotoxic insults mediated by overactivation of their AMPA ionotropic glutamate receptors. Phenolic compounds, which are widely distributed in fruits and vegetables, received the great attention of scientists due to their antioxidant activities and free radical scavenging abilities. Chlorogenic acid (CGA) has been demonstrated to possess potent neuroprotective activities against oxidative stress in various cellular models and pathological conditions. Hence, CGA protect against oxidative stress and excitotoxic insults mediated by AMPA receptors and that the protective mechanisms involve free radical scavenging, Ca2+ handling in the cytosol, and modulating antioxidant enzyme system. CGA was associated with the protein kinase A (PKC) signaling pathways transduction. Caspases and calpains have been studied as apoptotic mediators and cell death in this model of AMPA toxicity. Inhibitors of caspases initiators, caspases 1, 8, and 9, the upstream of caspase 3 effectors, have totally abrogated the protective activity of CGA. Inhibitors of calpains also totally abrogated the protective activity of CGA. In addition, a potential role for the CGA in inhibiting Bax in oligodendrocyte cell model undergoing AMPA is inducing excitotoxic death. Our results indicate that CGA exhibits a protective potential via antioxidant and apoptosis caspases and calpains dependent against AMPA-mediated excitotoxicity, and these finding indicate that CGA is able to be a good candidate for preventive approach for neurodegenerative disorders associated with loss and damage in oligodendrocytes and AMPA-mediated excitotoxicity.









Similar content being viewed by others
Abbreviations
- CGA:
-
Chlorogenic acid
- AMPA:
-
Alpha-amino-3-hydroxy-5-methyl-4 isoxazolepropionate
- NMDA:
-
N-methyl-D-aspartate
- ROS:
-
Reactive oxygen species
- CNS:
-
Central nervous system
- SNP:
-
System nervous peripheral
- Trolox:
-
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
- DCF:
-
Dichlorofluorescein
- FDA:
-
Fluorescein diacetate
- H2O2 :
-
Hydrogen peroxide
- PKA:
-
Protein kinase A
- PKC:
-
Protein kinase C
- PLC:
-
Phospholipase C
- MAPK:
-
Mitogen apoptotic protein kinase
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- DCF:
-
2′,7′-dichlorofluorescein
- PBS:
-
Phosphate-buffered saline
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- DMSO:
-
Dimethyl sulfoxide
- HBSS:
-
Hank’s balanced salt solution
- EDTA:
-
Ethylenediaminetetraacetic acid
- DNA:
-
Deoxyribonucleic acid
- ALS:
-
Amyotrophic lateral sclerosis
- PD:
-
Parkinson’s disease
- AD:
-
Alzheimer’s disease
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alberdi E, Sanchez-Gomez MV, Marino A, Matute C (2002) Ca2+influx through AMPA or kainate receptors alone is sufficient to initiate excitotoxicity in cultured oligodendrocytes. Neurobiol Dis 92:234–243
Alkon DL, Sun MK, Nelson TJ (2007) PKC signalling deficits: a mechanistic hypothesis for the origins of Alzheimer’s disease. Trends Pharmacol Sci 28:51–60
Almazon G, Mckay R, Cohen R (1999) Cyclic APMA regulates PDGF-stimulated signal transduction and differentiation of an immortalized optic-nerve-derived cell line. J Exp Biol 202:461–473
Andjelkovic M, Van J, Camp B, De Meulenaer G, Depaemelaere G, Cocaciu C, Verloo M, Verhe R (2006) Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem 98:23–31
Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC (1992) Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70(1):31–46
Battaini F (2001) Protein kinase C isoforms as therapeutic targets in nervous system disease states. Pharmacol Res 44(5):353–361
Bevers MB, Neumar RW, Cereb J (2008) Mechanistic role of calpains in postischemic neurodegeneration. Blood Flow Metab 28:655–673
Blandini F, Greenamyre JT, Nappi G (1996) The role of glutamate in the pathophysiology of Parkinson’s disease. Funct Neurol 11:3–15
Brown JE, Khodr H, Hider RC, Rice-Evans C (1998) Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochem J 330(3):1173–1178
Butt AM, Pugh M, Hubbard P, James G (2004) Functions of optic nerve glia: axoglial signalling in physiology and pathology. Eye 18:1110–1121
Campos-Esparza MR, Sanchez-Gomez LV, Matute C (2009) Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium 45:358–368
Chen S, Wu K, Knox R (2000) Structure-function studies of DT-diaphorase (NQO1) and NRH:quinone oxidoreductase (NQO2). Free Radic Biol Med 29:276–284
Connor JR, Menzies SL (1996) Relationship of iron to oligodendrocytes and myelination. Glia 17(2):83–93
Dai SM, Shan ZZ, Nakamura H (2006) Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum 54:818–831
Dailey A, Dailey H (2002) Identification of [2Fe-2S] clusters in microbial ferrochelatases. J Bacteriol 184(9):2460–2464
Dong H, Fazzaro A, Xiang C, Korsmeyer SJ, Jacquin MF, McDonald JW (2003) Enhanced oligodendrocyte survival after spinal cord injury in Bax-deficient mice and mice with delayed Wallerian degeneration. J Neurosci 23:8682–8691
Dos Santos MD, Almeida MC, Lopes NP, De Souza G (2006) Evaluation of the antiinflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull 29:2236–2240
Earnshaw XC, Martins LM, Kaufmann (1999) Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68:383–424
Fantuzzi G, Zheng H, Faggini R, Benigni F, Ghezzi P, Sipe JD, Shaw AR, Dinarello CA (1996) Effect of endotoxin in IL-1b-deficient mice. J Immunol 157:291–296
Foley S, Navaratnam S, McGarvey DJ, Land EJ, Truscott TG, Rice-Evans CA (1999) Singlet oxygen quenching and the redox properties of hydroxycinnamic acids. Free Radic Biol Med 26:1202–1208
Grosso G, Stepaniak U, Topor-Madry R, Szafraniec K, Pajak A (2014) Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 30:1398–1403
Gu C, Cassaccia-Bonnefil P, Srinivasan A, Chao MV (1999) Oligodendrocyte apoptosis mediated by caspase activation. J Neurosci 19:3043–3049
Halliwell B, Gutteridge JMC (1998) Free radicals in biology and medicine. Oxford University Press, Oxford
Ibarretexe G, Sanchez-Gomez, Campos-Esparza MR (2006) Differential oxidative stress in oligodendrocytes and neurons after excitotoxic insults and protection by natural polyphenols. Glia 53:201–211
Kim J, Lee S, Shim J, Kim HW, Kim J, Young JJ, Yang H, Park J, Choi SH, Yoon JH, Lee KW, Lee HJ (2012) Caffeinated coffee, decaffeinated coffee, and the phenolic phytochemical chlorogenicacid up-regulate NQO1 expression and prevent H2O2-induced apoptosis in primary cortical neurons. Neurochem Int 60:466–474
Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, Park YI, Lee CK, Lee YB, Lee SY, Jang CG (2010) Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via antiacetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol 649:210–217
Leardi A, Caraglia M, Selleri C, Pepe S, Pizzi C, Notaro R, Fabbrocini A, de Lorenzo S, MusicÒ M, Abbruzzese A, Bianco AR, Tagliaferri P (1998) Desferioxamine increases iron depletion and apoptosis induced by ara-C of human myeloid leukaemic cells. Br J Haematol 102:746–752
Lees GJ (2000) Pharmacology of AMPA/kainate receptor ligands and their therapeutic potential in neurological and psychiatric disorders. Drugs 59(1):33–78
Li P, Allen H, Banerjee S, Franklin S, Herzog L, Jonston C, Mcdowell J, Paskind M, Rodan L, Salfeld J, Tracey D, Wardwell S, Wei FY, Wong W, Seshardi T (1995) Mice deficient in IL-1b-converting enzyme are defective in production of mature IL-1b and resistant to endotoxic shock. Cell 80:401–411
Liochev SI, Fridovich I (1999) Superoxide and iron: partners in crime. IUBMB Life 48(2):157–161
Maher PJ (2001) How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J Neurosci 21(9):2929–2938
Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25:103–126
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signalling for suicide and survival. J Cell Physiol 192:1–15
Matute C, Alberdi E, Domerq M, Perez-Cerda F, Perez-Samarin A, Sanchez-Gomez MV (2001) The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci 24:224–230
Matute C, Alberdi E, Domercq M, Sánchez-Gómez MV, Pérez-Samartín A, Rodríguez-Antigüedad A, Pérez-Cerdá F (2007) Excitotoxic damage to white matter. J Anat 210(6):693–702
McCord JM, Boyle JA, Day ED, Rizzolo L, Salin ML (1977) In: Michelson AM, McCord JM, Fridovich I (eds) Superoxide dismutases. Academic Press, London, pp 129–138
Mikami Y, Yamazawa T (2015) Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci 139:69–74
Misra HP, Fridovich I (1972) The role of superoxide anion in autoxidation of epinephrine and a simple assay for SOD. J Biol Chem 247:3170–3317
Nagata S (1997) Apoptosis from death factor. Cell 85:355–365
Naveeda M, Hejazic V, Abbasa M, Kambohd AA, Khane GJ, Shumzaidf M, Ahmadg F, Babazadehh D, Fangi XF, Modarresi-Ghazanij F, Huab LH, Xiao Hui Z (2018) Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed Pharmacother 97:67
Ness JK, Scaduto RC Jr, Wood TL (2004) IGF-I prevents glutamate-mediated bax translocation and cytochrome C release in O4+ oligodendrocyte progenitors. Glia 46:183–194
Pang Y, Zheng B, Fan LW, Rhodes PG, Cai Z (2007) IGF-1 protects oligodendrocyte progenitors against TNFα-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia 55:1099–1107
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Rebai O, Belkhir M, Sanchez Gomez MV, Matute C, Fattouch S, Amri M (2017) Differential molecular targets for neuroprotective effect of chlorogenic acid and its related compounds against glutamate induced excitotoxicity and oxidative stress in rat cortical neurons. Neurochem Res 38(9)
Rice-Evans CA, Miller NJ, Bolwell PG, Broacgay PM, Pridham JB (1995) The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res 22:375–383
Rice-Evans CA, Miller JM, Paganga G (1996) Structure-antioxidant activity relationship of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Robinson KM, Janes MS, Pechar M, Monette JS, RRoss MF, Hagen TM (2006) Selective imaging of super oxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A 103:15038–15043
Ruvolo PP, Deng X, Carr BK, May WS (1998) A functional role for mitochondrial protein kinase in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem 273:25436–25442
Sánchez-Gómez MV, Matute C (1999) AMPA and kainate receptors each mediate excitotoxicity in oligodendroglial cultures. Neurobiol Dis 6(6):475–485
Sanchez-Gomez MV, Alberdi E, Ibarretxe G, Torre I, Matute C (2003) Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainate receptors. J Neurosci 23:9519–9528
Sanchez-Gomez MV, Alberdi E, Perez-Navarro E, Alberch J, Matute C (2011) Bax and calpain mediate excitotoxic oligodendrocyte death induced by activation of both AMPA and kainate receptors. J Neurosci 31:2996–3006
Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini R (2002) Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol. Pharm 3:554–561
Simonishvili S, Jain MR, Li H, Levison SW, Wood TL (2013) Identification of Bax-interacting proteins in oligodendrocyte progenitors during glutamate excitotoxicity and perinatal hypoxia–ischemia. ASN Neuro 5(5):e00131
Smith KJ, Kapoor R, Felts PA (1999) Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol 9:69–92
Sroka Z, Cisowski W (2003) Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol 41:753–758
Szwajgier D, Borowiec K, Pustelniak K (2017a) The neuroprotective effects of phenolic acids: molecular mechanism of action. Nutrients 9:477
Szwajgier D, Borowiec K, Pustelniak K (2017b) The neuroprotective effects of phenolic acids: molecular mechanism of action. Nutrients 9:477
Thornberry NA, Lazebnik Y (2008) Caspases: enemies within. Science 281:1312–1316
Van Acker SA, van den Berg DJ, Tromp MN et al (1996) Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med 20(3):331–342
Verkhratsky A, Steinhauser S (2000) Ion channels in glial cells. Brain Res 32:380–412
Voss OP, Milne S, Sharkey J, O’Neill MJ, McCulloch J (2007) Molecular mechanisms of neurite growth with AMPA receptor potentiation. Neuropharmacology 52:590–597
Welch KD, Davis TZ, Aust SD (2002) Iron autoxidation and free radical generation: effects of buffers, ligands, and chelators. Arch Biochem Biophys 397:360–369
Wu JM, Chen HX, Li H, Tang Y, Yang L, Cao SS, Qin DL (2016) Antidepressant potential of chlorogenic acid-enriched extract from Eucommia ulmoides Oliver bark with neuron protection and promotion of serotonin release through enhancing synapsin I expression. Molecules 21(3):260
Youdim MBH, Riederer P (2003) Iron in normal and pathological brain diseases. In: Smith B, Adleman G (eds) Encyclopedia of neuroscience. Elsevier, Amsterdam
Zamora-Ros R, Knaze V, Rothwell JA, Hémon B, Moskal A, Overvad K, Tjønneland A, Kyro C, Fagherazzi G, Boutron-Ruault MC et al (2016) Dietary polyphenol intake in Europe: the European prospective investigation into cancer and nutrition (EPIC) study. Eur J Nutr 55:1359–1375
Zarate CA Jr, Manji HK (2008) The role of AMPA receptor modulation in the treatment of neuropsychiatric diseases. Exp Neurol 211(1):7–10
Zhishen J, Mengcheng T, Jianming W (1999) The determination of falconoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Acknowledgments
We would like to thank Professor Matute Carlos and Sanchez-Gomez Maria Victoria as well as all the members of the Departamento de Neurociencias, Facultad de Medicina y Odontologia, Universidad Del Paıs Vasco, Leioa, Vizcaya, Spain, for their supportive advices.
Funding
This work was supported by the Research Unit 00-UR-08-01University of Sciences Tunis and by a grant from the Tunisian Ministry of Higher Education and Scientific Research Tunisia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rebai, O., Amri, M. Chlorogenic Acid Prevents AMPA-Mediated Excitotoxicity in Optic Nerve Oligodendrocytes Through a PKC and Caspase-Dependent Pathways. Neurotox Res 34, 559–573 (2018). https://doi.org/10.1007/s12640-018-9911-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9911-5