Abstract
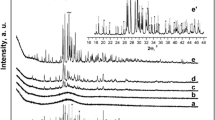
The effect of yttria on the solid reaction mechanism of a CaHPO4·2H2O + CaCO3 system at different temperatures was experimentally studied. The samples with and without yttria were subjected to thermogravimetric/differential scanning calorimetry measurement. The samples were heat treated at the temperatures corresponding to the peaks on the DSC spectra, and the resulted phase compositions were identified by X-ray diffraction. The transformation mechanism was deduced by comparing the phases obtained at different temperatures. The results show that the transformations at below 1073 K are not affected by yttria, but all those at above 1073 K are completely altered. The formation temperature of hydroxyapatite decreases by 134 K, and the decomposition temperature increases by 38 K. The polymorphous transformation of Ca3(PO4)2 from β phase to α phase increases by 47 K. The thermodynamic properties of the transformations at above 1073 K are also modified by the addition of yttria; that is, the endothermal peaks are substituted by exothermal peaks.
Similar content being viewed by others
References
Pinchuk N.D. and Ivanchenko L.A., Making calcium phosphate biomaterials, Powder Metall. Met. Ceram., 2003, 42(7–8): 357.
Ergun C., Webster T.J., Bizios R., and Doremus R.H., Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. I. Structure and microstructure, J. Biomed. Mater. Res., 2002, 59(2): 305.
Miyaji F., Kono Y., and Suyama Y., Formation and structure of zinc-substituted calcium hydroxyapatite, Mater. Res. Bull., 2005, 40(2): 209.
Suchanek W.L., Byrappa K., Shuk P., Riman R.E., Janas V.F., and TenHuisen K.S., Preparation of magnesium-substituted hydroxyapatite powders by the mechanochemical-hydrothermal method, Biomaterials, 2004, 25(19): 4647.
Kim S.R., Lee J.H., Kim Y.T., Riu D.H., Jung S.J., Lee Y.J., Chung S.C., and Kim Y.H., Synthesis of Si, Mg substituted hydroxyapatites and their sintering behaviors, Biomaterials, 2003, 24(8): 1389.
Webster T.J., Ergun C., Doremus R. and Bizios H.,R., Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. II. Mechanisms of osteoblast adhesion, J. Biomed. Mater. Res., 2002, 59(2): 312.
Sato M., Sambito M.A., Aslani A., Kalkhoran N.M., Slamovich E.B., and Webster T.J., Increased osteoblast functions on undoped and yttrium-doped nanocrystalline hydroxyapatite coatings on titanium, Biomaterials, 2006, 27(11): 2358.
Liu Q., Zou L., Zheng M., and Dong C., Effect of rare earth Y2O3 content of microstructure of gradient bioceramic composite coating produced by wide-band laser cladding, Key Eng. Mater., 2005, 288–289: 351.
Gao J.C., Zhang Y.P., Wen J., and Wang Y., Laser surface coating of RE bioceramic layer on TC4, Trans. Nonferrous. Met. Soc. China, 2000, 10(4): 477.
Samuskevich V.V., Belous N.K., and Samuskevich L.N., Sequence of solid-state transformations during heat treatment of CaCO3 + CaHPO4 mixtures, Inorg. Mater., 2003, 39(5): 520.
Fu L., Khor K.A., and Lim J.P., Yttria stabilized zirconia reinforced hydroxyapatite, Surf. Coat. Technol., 2000, 127(1): 66.
Miao X., Chen Y., Guo H., and Khor K.A., Spark plasma sintered hydroxyapatite-yttria stabilized zirconia composites, Ceram. Int., 2004, 30(7): 1793.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Gao, J., Hu, J. et al. Solid reaction mechanism of CaHPO4·2H2O + CaCO3 with and without yttria. Rare Metals 28, 77–81 (2009). https://doi.org/10.1007/s12598-009-0015-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-009-0015-5