Abstract
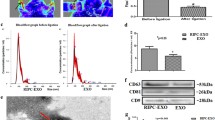
Exosomes are small-sized vesicles that can be released from cells into the serum. Exosomes play important roles in regulating many biological processes including cell proliferation, apoptosis, cell cycle, and metabolism. However, the roles and mechanisms of plasma exosomes in the apoptosis of rat H9C2 cardiomyocytes are largely unknown. In this study, we isolated plasma exosomes as confirmed by the marker protein CD63. Using flow cytometry and western blot analysis, we found that exosomes attenuated hydrogen peroxide (H2O2)-induced apoptosis and improved survival of rat H9C2 cardiomyocytes. Furthermore, the anti-apoptosis effects of serum exosomes in rat H9C2 cardiomyocytes were mediated by the activation of ERK1/2 signaling pathway. These data indicated that plasma exosomes had the protective effects against cardiomyocyte apoptosis and might be a novel therapy strategy for myocardial injury.




Similar content being viewed by others
References
Orogo, A. M., & Gustafsson, A. B. (2013). Cell death in the myocardium: my heart won’t go on. IUBMB Life, 65(8), 651–656. https://doi.org/10.1002/iub.1180
Olivetti, G., Abbi, R., Quaini, F., Kajstura, J., Cheng, W., Nitahara, J. A., et al. (1997). Apoptosis in the failing human heart. The New England Journal of Medicine, 336(16), 1131–1141. https://doi.org/10.1056/NEJM199704173361603
Lefer, D. J., & Granger, D. N. (2000). Oxidative stress and cardiac disease. The American Journal of Medicine, 109(4), 315–323.
Agarwal, U., George, A., Bhutani, S., Ghosh-Choudhary, S., Maxwell, J. T., Brown, M. E., et al. (2017). Experimental, systems, and computational approaches to understanding the microRNA-mediated reparative potential of cardiac progenitor cell-derived exosomes from pediatric patients. Circulation Research, 120(4), 701–712. https://doi.org/10.1161/CIRCRESAHA.116.309935.
Angelini, F., Ionta, V., Rossi, F., Pagano, F., Chimenti, I., Messina, E., et al. (2016). Exosomes isolation protocols: facts and artifacts for cardiac regeneration. Frontiers in Bioscience (Scholar Edition), 8, 303–311.
Kishore, R., & Khan, M. (2017). Cardiac cell-derived exosomes: changing face of regenerative biology. European Heart Journal, 38(3), 212–215. https://doi.org/10.1093/eurheartj/ehw324
Singla, D. K. (2016). Stem cells and exosomes in cardiac repair. Current Opinion in Pharmacology, 27, 19–23. https://doi.org/10.1016/j.coph.2016.01.003
Song, J., Chen, X., Wang, M., Xing, Y., Zheng, Z., & Hu, S. (2014). Cardiac endothelial cell-derived exosomes induce specific regulatory B cells. Scientific Reports, 4, 7583. https://doi.org/10.1038/srep07583
Wang, C., Zhang, C., Liu, L., A, X., Chen, B., Li, Y., et al. (2017). Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Molecular Therapy, 25(1), 192–204. https://doi.org/10.1016/j.ymthe.2016.09.001.
Zhang, Z., Yang, J., Yan, W., Li, Y., Shen, Z., & Asahara, T. (2016). Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. Journal of the American Heart Association, 5(1). https://doi.org/10.1161/JAHA.115.002856.
Khalyfa, A., & Gozal, D. (2014). Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. Journal of Translational Medicine, 12, 162. https://doi.org/10.1186/1479-5876-12-162
Owens 3rd, A. P., & Mackman, N. (2011). Microparticles in hemostasis and thrombosis. Circulation Research, 108(10), 1284–1297. https://doi.org/10.1161/CIRCRESAHA.110.233056
Loyer, X., Vion, A. C., Tedgui, A., & Boulanger, C. M. (2014). Microvesicles as cell-cell messengers in cardiovascular diseases. Circulation Research, 114(2), 345–353. https://doi.org/10.1161/CIRCRESAHA.113.300858
de Jong, O. G., Verhaar, M. C., Chen, Y., Vader, P., Gremmels, H., Posthuma, G., et al. (2012). Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. Journal of Extracellular Vesicles, 1. https://doi.org/10.3402/jev.v1i0.18396.
Li, C., Pei, F., Zhu, X., Duan, D. D., & Zeng, C. (2012). Circulating microRNAs as novel and sensitive biomarkers of acute myocardial infarction. Clinical Biochemistry, 45(10–11), 727–732. https://doi.org/10.1016/j.clinbiochem.2012.04.013
Kuwabara, Y., Ono, K., Horie, T., Nishi, H., Nagao, K., Kinoshita, M., et al. (2011). Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circulation. Cardiovascular Genetics, 4(4), 446–454. https://doi.org/10.1161/CIRCGENETICS.110.958975
D'Alessandra, Y., Devanna, P., Limana, F., Straino, S., Di Carlo, A., Brambilla, P. G., et al. (2010). Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. European Heart Journal, 31(22), 2765–2773. https://doi.org/10.1093/eurheartj/ehq167
Cheng, Y., Wang, X., Yang, J., Duan, X., Yao, Y., Shi, X., et al. (2012). A translational study of urine miRNAs in acute myocardial infarction. Journal of Molecular and Cellular Cardiology, 53(5), 668–676. https://doi.org/10.1016/j.yjmcc.2012.08.010
Bi, S., Wang, C., Jin, Y., Lv, Z., Xing, X., & Lu, Q. (2015). Correlation between serum exosome derived miR-208a and acute coronary syndrome. International Journal of Clinical and Experimental Medicine, 8(3), 4275–4280.
Goren, Y., Kushnir, M., Zafrir, B., Tabak, S., Lewis, B. S., & Amir, O. (2012). Serum levels of microRNAs in patients with heart failure. European Journal of Heart Failure, 14(2), 147–154. https://doi.org/10.1093/eurjhf/hfr155
Cheow, E. S., Cheng, W. C., Lee, C. N., de Kleijn, D., Sorokin, V., & Sze, S. K. (2016). Plasma-derived extracellular vesicles contain predictive biomarkers and potential therapeutic targets for myocardial ischemic (MI) injury. Molecular & Cellular Proteomics, 15(8), 2628–2640. https://doi.org/10.1074/mcp.M115.055731
Vicencio, J. M., Yellon, D. M., Sivaraman, V., Das, D., Boi-Doku, C., Arjun, S., et al. (2015). Plasma exosomes protect the myocardium from ischemia-reperfusion injury. Journal of the American College of Cardiology, 65(15), 1525–1536. https://doi.org/10.1016/j.jacc.2015.02.026
Milerova, M., Charvatova, Z., Skarka, L., Ostadalova, I., Drahota, Z., Fialova, M., et al. (2010). Neonatal cardiac mitochondria and ischemia/reperfusion injury. Molecular and Cellular Biochemistry, 335(1–2), 147–153. https://doi.org/10.1007/s11010-009-0251-x
Abas, L., Bogoyevitch, M. A., & Guppy, M. (2000). Mitochondrial ATP production is necessary for activation of the extracellular-signal-regulated kinases during ischaemia/reperfusion in rat myocyte-derived H9c2 cells. The Biochemical Journal, 349(Pt 1), 119–126.
Muscari, C., Gamberini, C., Bonafe, F., Giordano, E., Bianchi, C., Lenaz, G., et al. (2004). Evaluation of cellular energetics by the Pasteur effect in intact cardiomyoblasts and isolated perfused hearts. Molecular and Cellular Biochemistry, 258(1–2), 91–97.
Kuznetsov, A. V., Javadov, S., Sickinger, S., Frotschnig, S., & Grimm, M. (2015). H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochimica et Biophysica Acta, 1853(2), 276–284. https://doi.org/10.1016/j.bbamcr.2014.11.015
Bienert, G. P., Schjoerring, J. K., & Jahn, T. P. (2006). Membrane transport of hydrogen peroxide. Biochimica et Biophysica Acta, 1758(8), 994–1003. https://doi.org/10.1016/j.bbamem.2006.02.015
Han, H., Long, H., Wang, H., Wang, J., Zhang, Y., & Wang, Z. (2004). Progressive apoptotic cell death triggered by transient oxidative insult in H9c2 rat ventricular cells: a novel pattern of apoptosis and the mechanisms. American Journal of Physiology. Heart and Circulatory Physiology, 286(6), H2169–H2182. https://doi.org/10.1152/ajpheart.00199.2003
Ma, J., Zhao, Y., Sun, L., Sun, X., Zhao, X., Sun, X., et al. (2017). Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Translational Medicine, 6(1), 51–59. https://doi.org/10.5966/sctm.2016-0038
Morelli, A. E. (2006). The immune regulatory effect of apoptotic cells and exosomes on dendritic cells: its impact on transplantation. American Journal of Transplantation, 6(2), 254–261. https://doi.org/10.1111/j.1600-6143.2005.01197.x
Wang, Y., Zhang, L., Li, Y., Chen, L., Wang, X., Guo, W., et al. (2015). Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. International Journal of Cardiology, 192, 61–69. https://doi.org/10.1016/j.ijcard.2015.05.020
Xiao, J., Pan, Y., Li, X. H., Yang, X. Y., Feng, Y. L., Tan, H. H., et al. (2016). Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death & Disease, 7(6), e2277. https://doi.org/10.1038/cddis.2016.181
Yu, B., Kim, H. W., Gong, M., Wang, J., Millard, R. W., Wang, Y., et al. (2015). Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. International Journal of Cardiology, 182, 349–360. https://doi.org/10.1016/j.ijcard.2014.12.043
Bei, Y., Xu, T., Lv, D., Yu, P., Xu, J., Che, L., et al. (2017). Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Research in Cardiology, 112(4), 38. https://doi.org/10.1007/s00395-017-0628-z
Liang, Y., & Sahoo, S. (2015). Exosomes explosion for cardiac resuscitation. Journal of the American College of Cardiology, 66(6), 612–615. https://doi.org/10.1016/j.jacc.2015.06.1302
Tong, Z., Jiang, B., Wu, Y., Liu, Y., Li, Y., Gao, M., et al. (2015). MiR-21 protected cardiomyocytes against doxorubicin-induced apoptosis by targeting BTG2. International Journal of Molecular Sciences, 16(7), 14511–14525. https://doi.org/10.3390/ijms160714511
Peche, H., Renaudin, K., Beriou, G., Merieau, E., Amigorena, S., & Cuturi, M. C. (2006). Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. American Journal of Transplantation, 6(7), 1541–1550. https://doi.org/10.1111/j.1600-6143.2006.01344.x
Pironti, G., Strachan, R. T., Abraham, D., Mon-Wei Yu, S., Chen, M., Chen, W., et al. (2015). Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation, 131(24), 2120–2130. https://doi.org/10.1161/CIRCULATIONAHA.115.015687
Prathipati, P., Nandi, S. S., & Mishra, P. K. (2017). Stem cell-derived exosomes, autophagy, extracellular matrix turnover, and miRNAs in cardiac regeneration during stem cell therapy. Stem Cell Reviews, 13(1), 79–91. https://doi.org/10.1007/s12015-016-9696-y
Emanueli, C., Shearn, A. I., Laftah, A., Fiorentino, F., Reeves, B. C., Beltrami, C., et al. (2016). Coronary artery-bypass-graft surgery increases the plasma concentration of exosomes carrying a cargo of cardiac microRNAs: an example of exosome trafficking out of the human heart with potential for cardiac biomarker discovery. PLoS One, 11(4), e0154274. https://doi.org/10.1371/journal.pone.0154274
Looze, C., Yui, D., Leung, L., Ingham, M., Kaler, M., Yao, X., et al. (2009). Proteomic profiling of human plasma exosomes identifies PPARgamma as an exosome-associated protein. Biochemical and Biophysical Research Communications, 378(3), 433–438. https://doi.org/10.1016/j.bbrc.2008.11.050
Moldovan, L., Batte, K., Wang, Y., Wisler, J., & Piper, M. (2013). Analyzing the circulating microRNAs in exosomes/extracellular vesicles from serum or plasma by qRT-PCR. Methods in Molecular Biology, 1024, 129–145. https://doi.org/10.1007/978-1-62703-453-1_10
Ye, W., Tang, X., Yang, Z., Liu, C., Zhang, X., Jin, J., et al. (2017). Plasma-derived exosomes contribute to inflammation via the TLR9-NF-kappaB pathway in chronic heart failure patients. Molecular Immunology, 87, 114–121. https://doi.org/10.1016/j.molimm.2017.03.011
Chen, M., Zsengeller, Z., Xiao, C. Y., & Szabo, C. (2004). Mitochondrial-to-nuclear translocation of apoptosis-inducing factor in cardiac myocytes during oxidant stress: potential role of poly(ADP-ribose) polymerase-1. Cardiovascular Research, 63(4), 682–688. https://doi.org/10.1016/j.cardiores.2004.04.018
Chen, Z., Ge, Y., & Kang, J. X. (2004). Down-regulation of the M6P/IGF-II receptor increases cell proliferation and reduces apoptosis in neonatal rat cardiac myocytes. BMC Cell Biology, 5, 15. https://doi.org/10.1186/1471-2121-5-15
Kunapuli, S., Rosanio, S., & Schwarz, E. R. (2006). “How do cardiomyocytes die?” apoptosis and autophagic cell death in cardiac myocytes. Journal of Cardiac Failure, 12(5), 381–391. https://doi.org/10.1016/j.cardfail.2006.02.002
Pei, Z. H., Chen, B. Y., Tie, R., Zhang, H. F., Zhao, G., Qu, P., et al. (2011). Infrasound exposure induces apoptosis of rat cardiac myocytes by regulating the expression of apoptosis-related proteins. Cardiovascular Toxicology, 11(4), 341–346. https://doi.org/10.1007/s12012-011-9126-y
Yang, Y., Li, C., Xiang, X., Dai, Z., Chang, J., Zhang, M., et al. (2014). Ursolic acid prevents endoplasmic reticulum stress-mediated apoptosis induced by heat stress in mouse cardiac myocytes. Journal of Molecular and Cellular Cardiology, 67, 103–111. https://doi.org/10.1016/j.yjmcc.2013.12.018
Li, C., Tian, J., Li, G., Jiang, W., Xing, Y., Hou, J., et al. (2010). Asperosaponin VI protects cardiac myocytes from hypoxia-induced apoptosis via activation of the PI3K/Akt and CREB pathways. European Journal of Pharmacology, 649(1–3), 100–107. https://doi.org/10.1016/j.ejphar.2010.08.060
Ling, S., Luo, R., Dai, A., Guo, Z., Guo, R., & Komesaroff, P. A. (2009). A pharmaceutical preparation of Salvia miltiorrhiza protects cardiac myocytes from tumor necrosis factor-induced apoptosis and reduces angiotensin II-stimulated collagen synthesis in fibroblasts. Phytomedicine, 16(1), 56–64. https://doi.org/10.1016/j.phymed.2008.09.008
Townsend, P. A., Scarabelli, T. M., Pasini, E., Gitti, G., Menegazzi, M., Suzuki, H., et al. (2004). Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB Journal, 18(13), 1621–1623. https://doi.org/10.1096/fj.04-1716fje
Das, A., Salloum, F. N., Xi, L., Rao, Y. J., & Kukreja, R. C. (2009). ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. American Journal of Physiology: Heart and Circulatory Physiology, 296(5), H1236–H1243. https://doi.org/10.1152/ajpheart.00100.2009
Das, A., Xi, L., & Kukreja, R. C. (2008). Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. Journal of Biological Chemistry, 283(43), 29572–29585. https://doi.org/10.1074/jbc.M801547200
Fryer, R. M., Hsu, A. K., & Gross, G. J. (2001). ERK and p38 MAP kinase activation are components of opioid-induced delayed cardioprotection. Basic Research in Cardiology, 96(2), 136–142.
Hu, Y., Chen, X., Pan, T. T., Neo, K. L., Lee, S. W., Khin, E. S., et al. (2008). Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflügers Archiv European Journal of Physiology, 455(4), 607–616. https://doi.org/10.1007/s00424-007-0321-4
Naitoh, K., Ichikawa, Y., Miura, T., Nakamura, Y., Miki, T., Ikeda, Y., et al. (2006). MitoKATP channel activation suppresses gap junction permeability in the ischemic myocardium by an ERK-dependent mechanism. Cardiovascular Research, 70(2), 374–383. https://doi.org/10.1016/j.cardiores.2006.01.023
Ottani, A., Galantucci, M., Ardimento, E., Neri, L., Canalini, F., Calevro, A., et al. (2013). Modulation of the JAK/ERK/STAT signaling in melanocortin-induced inhibition of local and systemic responses to myocardial ischemia/reperfusion. Pharmacological Research, 72, 1–8. https://doi.org/10.1016/j.phrs.2013.03.005
Thomas, C. J., Lim, N. R., Kedikaetswe, A., Yeap, Y. Y., Woodman, O. L., Ng, D. C., et al. (2015). Evidence that the MEK/ERK but not the PI3K/Akt pathway is required for protection from myocardial ischemia-reperfusion injury by 3′,4′-dihydroxyflavonol. European Journal of Pharmacology, 758, 53–59. https://doi.org/10.1016/j.ejphar.2015.03.054
Filippone, S. M., Samidurai, A., Roh, S. K., Cain, C. K., He, J., Salloum, F. N., et al. (2017). Reperfusion therapy with rapamycin attenuates myocardial infarction through activation of AKT and ERK. Oxidative Medicine and Cellular Longevity, 2017, 4619720. https://doi.org/10.1155/2017/4619720
Vivar, R., Humeres, C., Varela, M., Ayala, P., Guzman, N., Olmedo, I., et al. (2012). Cardiac fibroblast death by ischemia/reperfusion is partially inhibited by IGF-1 through both PI3K/Akt and MEK-ERK pathways. Experimental and Molecular Pathology, 93, 1), 1–1), 7. https://doi.org/10.1016/j.yexmp.2012.01.010
Wu, X., Xu, T., Li, D., Zhu, S., Chen, Q., Hu, W., et al. (2013). ERK/PP1a/PLB/SERCA2a and JNK pathways are involved in luteolin-mediated protection of rat hearts and cardiomyocytes following ischemia/reperfusion. PLoS One, 8(12), e82957. https://doi.org/10.1371/journal.pone.0082957
Zhang, J., Bian, H. J., Li, X. X., Liu, X. B., Sun, J. P., Li, N., et al. (2010). ERK-MAPK signaling opposes rho-kinase to reduce cardiomyocyte apoptosis in heart ischemic preconditioning. Molecular Medicine, 16(7–8), 307–315. https://doi.org/10.2119/molmed.2009.00121.
Funding
This work was supported by the grants from the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-12M-1-006), National Natural Science Foundation of China (81700228 to Y Xie, 81570332 to L Zhou) and the grant from Jiangsu Province’s Key Provincial Talents Program (ZDRCA2016019).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All human investigations conformed to the principles outlined in the Declaration of Helsinki and were approved by the institutional review committees of Nanjing Medical School. All participants provided written informed consent when they were enrolled in this study.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Rights and permissions
About this article
Cite this article
Li, P., Liu, Z., Xie, Y. et al. Serum Exosomes Attenuate H2O2-Induced Apoptosis in Rat H9C2 Cardiomyocytes via ERK1/2. J. of Cardiovasc. Trans. Res. 12, 37–44 (2019). https://doi.org/10.1007/s12265-018-9791-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-018-9791-3