Abstract
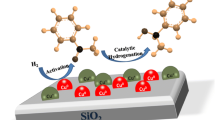
Cu/SiO2 catalysts that contain copper phyllosilicate, were successfully prepared using the ammonia-evaporation method. The catalysts were characterized via XRD, ICP, BET, FTIR, TPR, XPS, NH3-TPD and FTIR of Pyridine Adsorption techniques. The results demonstrated that the formation of the copper phyllosilicate species significantly affected the structural properties and caused the CuO nanoparticles to become highly dispersed, and the copper phyllosilicate would provide access to the Lewis acidic Cu+ species. It was found that the catalyst with a 23.7 wt% copper loading exhibited the best ethanol conversion and ethyl acetate selectivity. When compared to a catalyst with the same copper loading which was prepared with the impregnation method, the higher activity and selectivity of catalysts might be ascribed to the homogenous distribution of copper nanoparticles, which was the active site for the dehydrogenation, and the amount of Lewis acidic Cu+ sites active for esterification. The synergetic effect between the Cu0 and Lewis acidic sites was the key factor to achieve direct transformation of ethanol to ethyl acetate.

Cu/SiO2 catalysts, which contain copper phyllosilicate, were successfully prepared using the ammonia-evaporation method. Copper phyllosilicate species caused the CuO nanoparticles to become highly dispersed, and they would provide access to the Lewis acidic Cu+ species. Synergetic effect between Cu0 and Lewis acidic sites was the key factor for the reaction.







Similar content being viewed by others
References
Colley S W, Tabatabaei J, Waugh K C and Wood M A 2005 J. Catal. 236 21
Inui K, Kurabayashi T and Sato S 2002 Appl. Catal., A 237 53
Inui K, Kurabayashi T and Sato S 2002 J. Catal. 212 207
Inui K, Kurabayashi T, Sato S and Ichikawa N 2004 J. Mol. Catal. A: Chem. 216 147
Zonetti P C, Celnik J, Letichevsky S, Gaspar A B and Appel L G 2011 J. Mol. Catal. A: Chem. 334 29
Wang L X, Zhu W C, Zheng D F, Yu X, Cui J, Jia M, Zhang W X and Wang Z L 2010 Reac. Kinet. Mech. Cat. 101 365
Ji Chan Park H J L, Jung Up Bang, Kang Hyun Park and Hyunjoon Song 2009 Chem. Commun. 7345
Zhang B, Hui S, Zhang S, Ji Y, Li W and Fang D 2012 J. Nat. Gas Chem. 21 563
Che M, Cheng Z X and Louis C 1995 J. Am. Chem. Soc. 117 2008
Burattin P, Che M and Louis C 1997 J. Phys. Chem. B 101 7060
Fonseca M G and Airoldi C 2000 Thermochim. Acta 359 1
Lagadic I L, Mitchell M K and Payne B D 2001 Environ. Sci. Technol. 35 984
Mhamdi M, Marceau E, Khaddar-Zine S, Ghorbel A, Che M, Taarit Y and Villain F 2004 Catal. Lett. 98 135
Loaiza-Gil A, Arenas J, Villarroel M, Imbert F, del Castillo H and Fontal B 2005 J. Mol. Catal. A: Chem. 228 339
Schlegel M L and Manceau A 2006 Geochim. Cosmochim. Acta 70 901
Toupance T, Kermarec M and Louis C 2000 J. Phys. Chem. B 104 965
Toupance T, Kermarec M, Lambert J-F and Louis C 2002 J. Phys. Chem. B 106 2277
Huang Z, Cui F, Xue J, Zuo J, Chen J and Xia C 2010 J. Phys. Chem. C 114 16104
Chen L-F, Guo P-J, Qiao M-H, Yan S-R, Li H-X, Shen W, Xu H-L and Fan K-N 2008 J. Catal. 257 172
Zhao L, Zhao Y, Wang S, Yue H, Wang B, Lv J and Ma X 2012 Ind. Eng. Chem. Res. 51 13935
Yin A, Guo X, Dai W-L and Fan K 2011 Catal. Commun. 12 412
Gong J, Yue H, Zhao Y, Zhao S, Zhao L, Lv J, Wang S and Ma X 2012 J. Am. Chem. Soc. 134 13922
Pu Z-Y, Liu X-S, Jia A-P, Xie Y-L, Lu J-Q and Luo M-F 2008 J. Phys. Chem. C 112 15045
Zhu X, Li X, Jia M, Liu G, Zhang W and Jiang D 2005 Appl. Catal., A 282 155
Su W G, Wang S G, Ying P L, Feng Z C and Li C 2009 J. Catal. 268 165
Chen C-S. Chen C-S, You J-H, Lin J-H and Chen Y-Y 2008 Catal. Commun. 9 2381
Zhu Y-Y, Wang S-R, Zhu L-J, Ge X-L, Li X-B and Luo Z-Y 2010 Catal. Lett. 135 275
Marchi A J, Fierro J L G, Santamaría J and Monzón A 1996 Appl. Catal., A 142 375
Ghodselahi T, Vesaghi M A, Shafiekhani A, Baghizadeh A and Lameii M 2008 Appl. Surf. Sci. 255 2730
Huang Z, Cui F, Xue J, Zuo J, Chen J and Xia C 2012 Catal. Today 183 42
Ji D H, Zhu W C, Wang Z L and Wang G 2007 Catal. Commun. 8 1891
Matter P H and Ozkan U S 2005 J. Catal. 234 463
Agrell J, Birgersson H, Boutonnet M, Melián-Cabrera I, Navarro R M and Fierro J L G 2003 J. Catal. 219 389
Chen X R, Ju Y H and Mou C Y 2007 J. Phys. Chem. C 111 18731
Turco M, Bagnasco G, Cammarano C, Senese P, Costantino U and Sisani M 2007 Appl. Catal., B 77 46
Zhang Y Q, Wang S J, Wang J W, Lou L L, Zhang C and Liu S X 2009 Solid State Sci. 11 1412
Zhang B, Zhu Y, Ding G, Zheng H and Li Y 2012 Appl. Catal., A 443 191
Chen L F, Noreña L E, Navarrete J and Wang J A 2006 Mater. Chem. Phys. 97 236
Jia A Z, Li J, Zhang Y Q, Song Y J and Liu S X 2008 Mater. Sci. Eng., C 28 1217
Tonner S P, Trimm D L, Wainwright M S and Cant N W 1984 Ind. Eng. Chem. Prod. Res. Dev. 23 384
Crivello M E, Pérez C F, Mendieta S N, Casuscelli S G, Eimer G A, Elías V R and Herrero E R 2008 Catal. Today 133 787
Zhu W C, Wang L X, Liu S Y and Wang Z 2008 React. Kinet. Catal. Lett. 93 93
Gong Y, Dou T, Kang S, Li Q and Hu Y 2009 Fuel Process. Technol. 90 122
Benaliouche F, Boucheffa Y and Thibault-Starzyk F 2012 Microporous Mesoporous Mater. 147 10
Acknowledgements
This work was supported by the Natural Science Foundation of Science and Technology Department of Jilin Province (20130101015JC), the open project supported by State Key Laboratory of Inorganic Synthesis and Preparative Chemistry of Jilin University, the Innovation Project for the Frontiers of Science and the new interdisciplinary project of Jilin University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
YU, X., ZHAI, S., ZHU, W. et al. The direct transformation of ethanol to ethyl acetate over Cu/SiO 2 catalysts that contain copper phyllosilicate. J Chem Sci 126, 1013–1020 (2014). https://doi.org/10.1007/s12039-014-0659-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0659-z