Abstract
Purpose
The molecular mechanisms underlying tumor growth in Cushing’s disease (CD) still remain a challenge. Moreover, clinical manifestations of CD may vary depending on hormonal activity; however, factors involved in the hormonal aggressiveness of adrenocorticotropic hormone (ACTH)-secreting pituitary tumors have not been fully clarified. We investigated the association between the expression of cellular markers regarding pituitary tumor progression and initial or postoperative hormone levels in patients with CD.
Methods
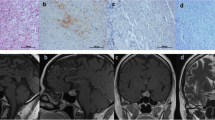
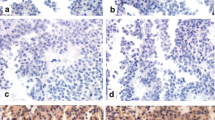
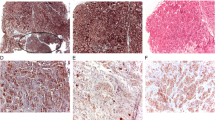
Tumor tissues from 28 corticotroph adenomas (female 26, male 2, mean age 39.21 ± 10.39 years) were subject to immunohistochemical study using the following antibodies: pituitary tumor-transforming gene 1 (PTTG1), cyclin D1, p16, p27, brahma related-gene 1 (Brg1), and Ki-67. We then analyzed the relationship between each cellular marker expression and hormone levels, including 24 h urinary free cortisol (UFC), plasma ACTH, and serum cortisol.
Results
PTTG1 and Ki-67 were expressed in 100% and 50% of patients, respectively. However, the levels did not reflect initial hormonal activity. The cyclin D1-negative group showed higher serum cortisol levels compared to the cyclin D1-positive group (p = 0.01). The 24 h UFC levels were significantly higher in the p27-negative group than in the p27-positive group (p = 0.04), whereas the Brg1-positive group revealed higher serum cortisol levels than in the Brg1-negative group (p = 0.02).
Conclusions
Although PTTG1 and Ki-67 play an essential role in developing ACTH-secreting tumors, cyclin D1, p27, and Brg1 may be better biomarkers to determine hormonal aggressiveness of the tumor. Further research is needed to understand the influence of cellular markers on hormonal activity in CD.


Similar content being viewed by others
References
M. De Martin, F. Pecori Giraldi, F. Cavagnini, Cushing’s disease. Pituitary 9, 279–287 (2006)
J.J. Acebes, J. Martino, C. Masuet, E. Montanya, J. Soler, Early post-operative ACTH and cortisol as predictors of remission in Cushing’s disease. Acta Neurochir. 149, 471–477 (2007). discussion 477-479
G. Aranda, J. Ensenat, M. Mora, M. Puig-Domingo, M.J. Martinez de Osaba, G. Casals, E. Verger, M.T. Ribalta, F.A. Hanzu, I. Halperin, Long-term remission and recurrence rate in a cohort of Cushing’s disease: the need for long-term follow-up. Pituitary 18, 142–149 (2015)
Z.K. Hassan-Smith, M. Sherlock, R.C. Reulen, W. Arlt, J. Ayuk, A.A. Toogood, M.S. Cooper, A.P. Johnson, P.M. Stewart, Outcome of Cushing’s disease following transsphenoidal surgery in a single center over 20 years. J. Clin. Endocrinol. Metab. 97, 1194–1201 (2012)
K.Y. Hur, J.H. Kim, B.J. Kim, M.S. Kim, E.J. Lee, S.W. Kim, Clinical guidelines for the diagnosis and treatment of Cushing’s disease in Korea. Endocrinol. Metab. 30, 7–18 (2015)
B.M. Biller, A.B. Grossman, P.M. Stewart, S. Melmed, X. Bertagna, J. Bertherat, M. Buchfelder, A. Colao, A.R. Hermus, L.J. Hofland, A. Klibanski, A. Lacroix, J.R. Lindsay, J. Newell-Price, L.K. Nieman, S. Petersenn, N. Sonino, G.K. Stalla, B. Swearingen, M.L. Vance, J.A. Wass, M. Boscaro, Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J. Clin. Endocrinol. Metab. 93, 2454–2462 (2008)
J.K. Lambert, L. Goldberg, S. Fayngold, J. Kostadinov, K.D. Post, E.B. Geer, Predictors of mortality and long-term outcomes in treated Cushing’s disease: a study of 346 patients. J. Clin. Endocrinol. Metab. 98, 1022–1030 (2013)
N. Hameed, C.G. Yedinak, J. Brzana, S.H. Gultekin, N.D. Coppa, A. Dogan, J.B. Delashaw, M. Fleseriu, Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing’s disease, the role of cortisol, ACTH assessment and immediate reoperation: a large single center experience. Pituitary 16, 452–458 (2013)
D. Lau, C. Rutledge, M.K. Aghi, Cushing’s disease: current medical therapies and molecular insights guiding future therapies. Neurosurg. Focus 38, E11 (2015)
C.G. Patil, D.M. Prevedello, S.P. Lad, M.L. Vance, M.O. Thorner, L. Katznelson, E.R. Laws Jr., Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J. Clin. Endocrinol. Metab. 93, 358–362 (2008)
N. Sonino, M. Zielezny, G.A. Fava, F. Fallo, M. Boscaro, Risk factors and long-term outcome in pituitary-dependent Cushing’s disease. J. Clin. Endocrinol. Metab. 81, 2647–2652 (1996)
S.L. Asa, S. Ezzat, The pathogenesis of pituitary tumors. Annu. Rev. Pathol. 4, 97–126 (2009)
J. Seltzer, C.E. Ashton, T.C. Scotton, D. Pangal, J.D. Carmichael, G. Zada, Gene and protein expression in pituitary corticotroph adenomas: a systematic review of the literature. Neurosurg. Focus 38, E17 (2015)
C.H. Kuo, S.R. Shih, H.Y. Li, S.C. Chen, P.J. Hung, F.Y. Tseng, T.C. Chang, Adrenocorticotropic hormone levels before treatment predict recurrence of Cushing’s disease. J. Formos. Med. Assoc. 116, 441–447 (2017)
F. Esposito, J.R. Dusick, P. Cohan, P. Moftakhar, D. McArthur, C. Wang, R.S. Swerdloff, D.F. Kelly, Clinical review: early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s disease. J. Clin. Endocrinol. Metab. 91, 7–13 (2006)
R.N. Clayton, D. Raskauskiene, R.C. Reulen, P.W. Jones, Mortality and morbidity in Cushing’s disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J. Clin. Endocrinol. Metab. 96, 632–642 (2011)
J.S. Lim, S.K. Lee, S.H. Kim, E.J. Lee, S.H. Kim, Intraoperative multiple-staged resection and tumor tissue identification using frozen sections provide the best result for the accurate localization and complete resection of tumors in Cushing’s disease. Endocrine 40, 452–461 (2011)
A. Ayala, A.J. Manzano, Detection of recurrent Cushing’s disease: proposal for standardized patient monitoring following transsphenoidal surgery. J. Neurooncol. 119, 235–242 (2014)
E.H. Oldfield, J.L. Doppman, L.K. Nieman, G.P. Chrousos, D.L. Miller, D.A. Katz, G.B. Cutler Jr., D.L. Loriaux, Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N. Engl. J. Med. 325, 897–905 (1991)
T.W. Noh, H.J. Jeong, M.K. Lee, T.S. Kim, S.H. Kim, E.J. Lee, Predicting recurrence of nonfunctioning pituitary adenomas. J. Clin. Endocrinol. Metab. 94, 4406–4413 (2009)
R. Gejman, B. Swearingen, E.T. Hedley-Whyte, Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum. Pathol. 39, 758–766 (2008)
J.R. Lindsay, E.H. Oldfield, C.A. Stratakis, L.K. Nieman, The postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing’s disease after transsphenoidal surgery. J. Clin. Endocrinol. Metab. 96, 2057–2064 (2011)
A.V. Pendharkar, E.S. Sussman, A.L. Ho, M.G. Hayden Gephart, L. Katznelson, Cushing’s disease: predicting long-term remission after surgical treatment. Neurosurg. Focus 38, E13 (2015)
E. Fernandez-Rodriguez, P.M. Stewart, M.S. Cooper, The pituitary-adrenal axis and body composition. Pituitary 12, 105–115 (2009)
H. Cushing, The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Bull. Johns. Hopkins Hosp. 50, 137 (1932)
M. Yaneva, K. Kalinov, S. Zacharieva, Mortality in Cushing’s syndrome: data from 386 patients from a single tertiary referral center. Eur. J. Endocrinol. 169, 621–627 (2013)
J. Drouin, S. Bilodeau, S. Vallette, Of old and new diseases: genetics of pituitary ACTH excess (Cushing) and deficiency. Clin. Genet. 72, 175–182 (2007)
L. Vilar, C. Freitas Mda, M. Faria, R. Montenegro, L.A. Casulari, L. Naves, O.D. Bruno, Pitfalls in the diagnosis of Cushing’s syndrome. Arq. Bras. Endocrinol. Metabol. 51, 1207–1216 (2007)
A.M. Robertson, A.P. Heaney, Molecular markers in pituitary tumors. Curr. Opin. Endocrinol. Diabetes Obes. 23, 324–330 (2016)
S.L. Asa, S. Ezzat, The pathogenesis of pituitary tumours. Nat. Rev. Cancer 2, 836–849 (2002)
S. Ezzat, S.L. Asa, Mechanisms of disease: The pathogenesis of pituitary tumors. Nat. Clin. Pract. Endocrinol. Metab. 2, 220–230 (2006)
S. Bilodeau, S. Vallette-Kasic, Y. Gauthier, D. Figarella-Branger, T. Brue, F. Berthelet, A. Lacroix, D. Batista, C. Stratakis, J. Hanson, B. Meij, J. Drouin, Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in Cushing disease. Genes Dev. 20, 2871–2886 (2006)
X. Liu, M. Feng, Y. Zhang, C. Dai, B. Sun, X. Bao, K. Deng, Y. Yao, R. Wang, Expression of Matrix Metalloproteinase-9, Pituitary Tumor Transforming Gene, High Mobility Group A 2, and Ki-67 in Adrenocorticotropic Hormone-Secreting Pituitary Tumors and Their Association with Tumor Recurrence. World Neurosurg. 113, e213–e221 (2018)
A. Wierinckx, C. Auger, P. Devauchelle, A. Reynaud, P. Chevallier, M. Jan, G. Perrin, M. Fevre-Montange, C. Rey, D. Figarella-Branger, G. Raverot, M.F. Belin, J. Lachuer, J. Trouillas, A diagnostic marker set for invasion, proliferation, and aggressiveness of prolactin pituitary tumors. Endocr. Relat. Cancer 14, 887–900 (2007)
M. Filippella, F. Galland, M. Kujas, J. Young, A. Faggiano, G. Lombardi, A. Colao, G. Meduri, P. Chanson, Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: a clinical and immunohistochemical study. Clin. Endocrinol. 65, 536–543 (2006)
B.W. Scheithauer, T.A. Gaffey, R.V. Lloyd, T.J. Sebo, K.T. Kovacs, E. Horvath, O. Yapicier, W.F. Young Jr., F.B. Meyer, T. Kuroki, D.L. Riehle, E.R. Laws Jr., Pathobiology of pituitary adenomas and carcinomas. Neurosurgery 59, 341–353 (2006). discussion 341-353
M. Musat, D.G. Morris, M. Korbonits, A.B. Grossman, Cyclins and their related proteins in pituitary tumourigenesis. Mol. Cell. Endocrinol. 326, 25–29 (2010)
M. Fedele, A. Fusco, Role of the high mobility group A proteins in the regulation of pituitary cell cycle. J. Mol. Endocrinol. 44, 309–318 (2010)
S. Jordan, K. Lidhar, M. Korbonits, D.G. Lowe, A.B. Grossman, Cyclin D and cyclin E expression in normal and adenomatous pituitary. Eur. J. Endocrinol. 143, R1–R6 (2000)
Y. Tani, N. Inoshita, T. Sugiyama, M. Kato, S. Yamada, M. Shichiri, Y. Hirata, Upregulation of CDKN2A and suppression of cyclin D1 gene expressions in ACTH-secreting pituitary adenomas. Eur. J. Endocrinol. 163, 523–529 (2010)
D. Reisman, E.A. Thompson, Glucocorticoid regulation of cyclin D3 gene transcription and mRNA stability in lymphoid cells. Mol. Endocrinol. 9, 1500–1509 (1995)
C. Attwooll, E. Lazzerini Denchi, K. Helin, The E2F family: specific functions and overlapping interests. EMBO J. 23, 4709–4716 (2004)
P.L. Dahia, R.C. Aguiar, J. Honegger, R. Fahlbush, S. Jordan, D.G. Lowe, X. Lu, R.N. Clayton, G.M. Besser, A.B. Grossman, Mutation and expression analysis of the p27/kip1 gene in corticotrophin-secreting tumours. Oncogene 16, 69–76 (1998)
M. Korbonits, H.S. Chahal, G. Kaltsas, S. Jordan, Y. Urmanova, Z. Khalimova, P.E. Harris, W.E. Farrell, F.X. Claret, A.B. Grossman, Expression of phosphorylatedp27(Kip1) protein and Jun activation domain-binding protein 1 in human pituitary tumors. J. Clin. Endocrinol. Metab. 87, 2635–2643 (2002)
A. Roussel-Gervais, S. Bilodeau, S. Vallette, F. Berthelet, A. Lacroix, D. Figarella-Branger, T. Brue, J. Drouin, Cooperation between cyclin E andp27(Kip1) in pituitary tumorigenesis. Mol. Endocrinol. 24, 1835–1845 (2010)
T. Zhang, B. Zhao, J. Li, C. Zhang, H. Li, J. Wu, S. Zhang, G. Hui, Pituitary gene expression differs in D-galactose-induced cell senescence and steroid-induced prolactinomas. Mol. Med. Rep. 11, 3027–3032 (2015)
M. Sapochnik, L.E. Nieto, M. Fuertes, E. Arzt, Molecular mechanisms underlying pituitary pathogenesis. Biochem. Genet. 54, 107–119 (2016)
Q. Wu, J.B. Lian, J.L. Stein, G.S. Stein, J.A. Nickerson, A.N. Imbalzano, The BRG1 ATPase of human SWI/SNF chromatin remodeling enzymes as a driver of cancer. Epigenomics 9, 919–931 (2017)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lim, J.S., Lee, MK., Choi, E. et al. Hormonal aggressiveness according to the expression of cellular markers in corticotroph adenomas. Endocrine 64, 147–156 (2019). https://doi.org/10.1007/s12020-018-1815-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1815-x