Abstract
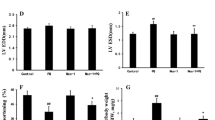
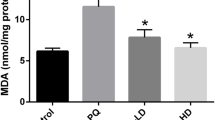
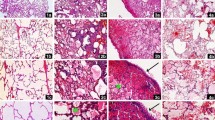
Paraquat (1,1’-dim ethyl-4-4’-bipyridinium dichloride), a highly toxic quaternary ammonium herbicide widely used in agriculture, exerts potent toxic prooxidant effects resulting in multi-organ failure including the lung and heart although the underlying mechanism remains elusive. Recent evidence suggests possible involvement of endothelin system in paraquat-induced acute lung injury. This study was designed to examine the role of endothelin receptor A (ETA) in paraquat-induced cardiac contractile and mitochondrial injury. Wild-type (WT) and cardiac-specific ETA receptor knockout mice were challenged to paraquat (45 mg/kg, i.p.) for 48 h prior to the assessment of echocardiographic, cardiomyocyte contractile and intracellular Ca2+ properties, as well as apoptosis and mitochondrial damage. Levels of the mitochondrial proteins for biogenesis and oxidative phosphorylation including UCP2, HSP90 and PGC1α were evaluated. Our results revealed that paraquat elicited cardiac enlargement, mechanical anomalies including compromised echocardiographic parameters (elevated left ventricular end-systolic and end-diastolic diameters as well as reduced factional shortening), suppressed cardiomyocyte contractile function, intracellular Ca2+ handling, overt apoptosis and mitochondrial damage. ETA receptor knockout itself failed to affect myocardial function, apoptosis, mitochondrial integrity and mitochondrial protein expression. However, ETA receptor knockout ablated or significantly attenuated paraquat-induced cardiac contractile and intracellular Ca2+ defect, apoptosis and mitochondrial damage. Taken together, these findings revealed that endothelin system in particular the ETA receptor may be involved in paraquat-induced toxic myocardial contractile anomalies possibly related to apoptosis and mitochondrial damage.




Similar content being viewed by others
References
Cristovao, A. C., Choi, D. H., Baltazar, G., Beal, M. F., & Kim, Y. S. (2009). The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxidants & Redox Signaling, 11, 2105–2118.
Jones, B. C., Huang, X., Mailman, R. B., Lu, L., & Williams, R. W. (2014). The perplexing paradox of paraquat: The case for host-based susceptibility and postulated neurodegenerative effects. Journal of Biochemical and Molecular Toxicology, 28, 191–197.
Blanco-Ayala, T., Anderica-Romero, A. C., & Pedraza-Chaverri, J. (2014). New insights into antioxidant strategies against paraquat toxicity. Free Radical Research, 48, 623–640.
Niso-Santano, M., Bravo-San Pedro, J. M., Gomez-Sanchez, R., Climent, V., Soler, G., Fuentes, J. M., & Gonzalez-Polo, R. A. (2011). ASK1 overexpression accelerates paraquat-induced autophagy via endoplasmic reticulum stress. Toxicological Sciences, 119, 156–168.
McCormack, A. L., Thiruchelvam, M., Manning-Bog, A. B., Thiffault, C., Langston, J. W., Cory-Slechta, D. A., & Di Monte, D. A. (2002). Environmental risk factors and Parkinson’s disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiology of Diseases, 10, 119–127.
Niso-Santano, M., Moran, J. M., Garcia-Rubio, L., Gomez-Martin, A., Gonzalez-Polo, R. A., Soler, G., & Fuentes, J. M. (2006). Low concentrations of paraquat induces early activation of extracellular signal-regulated kinase 1/2, protein kinase B, and c-Jun N-terminal kinase 1/2 pathways: Role of c-Jun N-terminal kinase in paraquat-induced cell death. Toxicological Sciences, 92, 507–515.
Peng, J., Mao, X. O., Stevenson, F. F., Hsu, M., & Andersen, J. K. (2004). The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. Journal of Biological Chemistry, 279, 32626–32632.
Dinis-Oliveira, R. J., Duarte, J. A., Sanchez-Navarro, A., Remiao, F., Bastos, M. L., & Carvalho, F. (2008). Paraquat poisonings: Mechanisms of lung toxicity, clinical features, and treatment. Critical Reviews in Toxicology, 38, 13–71.
Cherukuri, H., Pramoda, K., Rohini, D., Thunga, G., Vijaynarayana, K., Sreedharan, N., et al. (2014). Demographics, clinical characteristics and management of herbicide poisoning in tertiary care hospital. Toxicology International, 21, 209–213.
Wang, Q., Yang, L., Hua, Y., Nair, S., Xu, X., & Ren, J. (2014). AMP-activated protein kinase deficiency rescues paraquat-induced cardiac contractile dysfunction through an autophagy-dependent mechanism. Toxicological Sciences, 142, 6–20.
Fahim, M. A., Howarth, F. C., Nemmar, A., Qureshi, M. A., Shafiullah, M., Jayaprakash, P., & Hasan, M. Y. (2013). Vitamin E ameliorates the decremental effect of paraquat on cardiomyocyte contractility in rats. PLoS ONE, 8, e57651.
Song, C., Kan, B., Yu, G., Jian, X., Wang, J., & Sun, J. (2014). Acute paraquat poisoning with sinus bradycardia: A case report. Experimental and Therapeutic Medicine, 8, 1459–1462.
Dong, X. S., Xu, X. Y., Sun, Y. Q., Wei, L., Jiang, Z. H., & Liu, Z. (2013). Toll-like receptor 4 is involved in myocardial damage following paraquat poisoning in mice. Toxicology, 312, 115–122.
Ge, W., Zhang, Y., Han, X., & Ren, J. (2010). Cardiac-specific overexpression of catalase attenuates paraquat-induced myocardial geometric and contractile alteration: Role of ER stress. Free Radical Biology and Medicine, 49, 2068–2077.
Gonzalez-Polo, R. A., Rodriguez-Martin, A., Moran, J. M., Niso, M., Soler, G., & Fuentes, J. M. (2004). Paraquat-induced apoptotic cell death in cerebellar granule cells. Brain Research, 1011, 170–176.
McCormack, A. L., Atienza, J. G., Johnston, L. C., Andersen, J. K., Vu, S., & Di Monte, D. A. (2005). Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. Journal of Neurochemistry, 93, 1030–1037.
Alexi, T., Borlongan, C. V., Faull, R. L., Williams, C. E., Clark, R. G., Gluckman, P. D., & Hughes, P. E. (2000). Neuroprotective strategies for basal ganglia degeneration: Parkinson’s and Huntington’s diseases. Progress in Neurobiology, 60, 409–470.
Tawara, T., Fukushima, T., Hojo, N., Isobe, A., Shiwaku, K., Setogawa, T., & Yamane, Y. (1996). Effects of paraquat on mitochondrial electron transport system and catecholamine contents in rat brain. Archives of Toxicology, 70, 585–589.
Niso-Santano, M., Gonzalez-Polo, R. A., Bravo-San Pedro, J. M., Gomez-Sanchez, R., Lastres-Becker, I., Ortiz-Ortiz, M. A., et al. (2010). Activation of apoptosis signal-regulating kinase 1 is a key factor in paraquat-induced cell death: modulation by the Nrf2/Trx axis. Free Radical Biology and Medicine, 48, 1370–1381.
Lee, J. C., Park, C. Y., Choi, S. W., Kim, J. C., Lim, K. M., Kim, K., et al. (2008). Comparison of a pulsatile blood pump and a peristaltic roller pump during hemoperfusion treatment in a canine model of paraquat poisoning. Artificial Organs, 32, 541–546.
Wang, W. H., Zhang, H., Yu, Y. L., Jiang, J. H., & Xue, C. (2005). Correlation of plasma endothelin and multiple organ dysfunction syndrome caused by acute paraquat poisoning. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue, 17, 293–295.
Zhang, Z., Jian, X., Zhang, W., Wang, J., & Zhou, Q. (2013). Using Bosentan to treat paraquat poisoning-induced acute lung injury in rats. PLoS ONE, 8, e75943.
Finck, B. N., & Kelly, D. P. (2006). PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. The Journal of Clinical Investigation, 116, 615–622.
Schilling, J., & Kelly, D. P. (2011). The PGC-1 cascade as a therapeutic target for heart failure. Journal of Molecular and Cellular Cardiology, 51, 578–583.
Rey, B., Roussel, D., Romestaing, C., Belouze, M., Rouanet, J. L., Desplanches, D., et al. (2010). Up-regulation of avian uncoupling protein in cold-acclimated and hyperthyroid ducklings prevents reactive oxygen species production by skeletal muscle mitochondria. BMC physiology, 10, 5.
Latchman, D. S. (2001). Heat shock proteins and cardiac protection. Cardiovascular Research, 51, 637–646.
Mymrikov, E. V., & Haslbeck, M. (2015). Medical implications of understanding the functions of human small heat shock proteins. Expert Review of Proteomics, 12, 295–308.
Kedzierski, R. M., Grayburn, P. A., Kisanuki, Y. Y., Williams, C. S., Hammer, R. E., Richardson, J. A., et al. (2003). Cardiomyocyte-specific endothelin A receptor knockout mice have normal cardiac function and an unaltered hypertrophic response to angiotensin II and isoproterenol. Molecular and Cellular Biology, 23, 8226–8232.
Day, B. J., & Crapo, J. D. (1996). A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced lung injury in vivo. Toxicology and Applied Pharmacology, 140, 94–100.
Liang, L., Shou, X. L., Zhao, H. K., Ren, G. Q., Wang, J. B., Wang, X. H., et al. (2015). Antioxidant catalase rescues against high fat diet-induced cardiac dysfunction via an IKKbeta-AMPK-dependent regulation of autophagy. Biochimica et Biophysica Acta, 1852, 343–352.
Hu, N., Dong, M., & Ren, J. (2014). Hydrogen sulfide alleviates cardiac contractile dysfunction in an Akt2-knockout murine model of insulin resistance: role of mitochondrial injury and apoptosis. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 306, R761–R771.
Zhang, Y., Xu, X., Ceylan-Isik, A. F., Dong, M., Pei, Z., Li, Y., & Ren, J. (2014). Ablation of Akt2 protects against lipopolysaccharide-induced cardiac dysfunction: role of Akt ubiquitination E3 ligase TRAF6. Journal of Molecular and Cellular Cardiology, 74, 76–87.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Garcia-Garcia, A., Anandhan, A., Burns, M., Chen, H., Zhou, Y., & Franco, R. (2013). Impairment of Atg5-dependent autophagic flux promotes paraquat- and MPP(+)-induced apoptosis but not rotenone or 6-hydroxydopamine toxicity. Toxicological Sciences, 136, 166–182.
Gonzalez-Polo, R. A., Niso-Santano, M., Ortiz-Ortiz, M. A., Gomez-Martin, A., Moran, J. M., Garcia-Rubio, L., et al. (2007). Inhibition of paraquat-induced autophagy accelerates the apoptotic cell death in neuroblastoma SH-SY5Y cells. Toxicological Sciences, 97, 448–458.
Clark, D. G., McElligott, T. F., & Hurst, E. W. (1966). The toxicity of paraquat. British Journal of Industrial Medicine, 23, 126–132.
Bus, J. S., Aust, S. D., & Gibson, J. E. (1976). Paraquat toxicity: proposed mechanism of action involving lipid peroxidation. Environmental Health Perspectives, 16, 139–146.
Giri, S. N., Hollinger, M. A., & Schiedt, M. J. (1981). The effects of paraquat and superoxide dismutase on pulmonary vascular permeability and edema in mice. Archives of Environmental Health, 36, 149–154.
Li, Q., Yang, X., Sreejayan, N., & Ren, J. (2007). Insulin-like growth factor I deficiency prolongs survival and antagonizes paraquat-induced cardiomyocyte dysfunction: Role of oxidative stress. Rejuvenation Res, 10, 501–512.
Ceylan-Isik, A. F., Dong, M., Zhang, Y., Dong, F., Turdi, S., Nair, S., et al. (2013). Cardiomyocyte-specific deletion of endothelin receptor A rescues aging-associated cardiac hypertrophy and contractile dysfunction: role of autophagy. Basic Research in Cardiology, 108, 335.
Zhang, Y., Li, L., Hua, Y., Nunn, J. M., Dong, F., Yanagisawa, M., & Ren, J. (2012). Cardiac-specific knockout of ET(A) receptor mitigates low ambient temperature-induced cardiac hypertrophy and contractile dysfunction. Journal of Molecular Cell Biology, 4, 97–107.
Ren, J., Pulakat, L., Whaley-Connell, A., & Sowers, J. R. (2010). Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. Journal of Molecular Medicine (Berl), 88, 993–1001.
Zhang, Y., Xia, Z., La Cour, K. H., & Ren, J. (2011). Activation of Akt rescues endoplasmic reticulum stress-impaired murine cardiac contractile function via glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeation pore opening. Antioxidants & Redox Signaling, 15, 2407–2424.
Kim, H. R., Park, B. K., Oh, Y. M., Lee, Y. S., Lee, D. S., Kim, H. K., et al. (2006). Green tea extract inhibits paraquat-induced pulmonary fibrosis by suppression of oxidative stress and endothelin-l expression. Lung, 184, 287–295.
Acknowledgments
This work was supported in part by the Natural Science Foundation of China 81400198 and 81270171, as well as the Innovation Team Grant of Shaanxi Province (No. 2014KCT-20).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jiaxing Wang, Songhe Lu and Qijun Zheng have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, J., Lu, S., Zheng, Q. et al. Cardiac-Specific Knockout of ETA Receptor Mitigates Paraquat-Induced Cardiac Contractile Dysfunction. Cardiovasc Toxicol 16, 235–243 (2016). https://doi.org/10.1007/s12012-015-9331-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-015-9331-1