Abstract
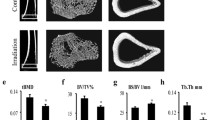
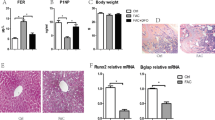
Patients with radiotherapy are at significant risks of bone loss and fracture. On the other hand, osteoporosis often occurs in disorders characterized by iron overload. Either ionizing radiation (IR) or iron overload alone has detrimental effects on bone metabolism, but their combined effects are not well defined. In this study, we evaluated the effects of IR on bone loss in an iron-overload mouse model induced by intraperitoneal injection of ferric ammonium citrate (FAC). In the present study, we found that IR additively aggravated iron overload induced by FAC injections. Iron overload stimulated hepcidin synthesis, while IR had an inhibitory effect and even inhibited the stimulatory effects of iron overload. Micro-CT analysis demonstrated that the loss of bone mineral density and bone volume, and the deterioration of bone microarchitecture were greatest in combined treatment group. Iron altered the responses of bone cells to IR. Iron enhanced the responses of osteoclasts to IR with elevated osteoclast differentiation, but did not affect osteoblast differentiation. Our study indicates that IR and iron in combination lead to a more severe impact on the bone homeostasis when compared with their respective effects. IR aggravated iron overload induced bone loss by heightened bone resorption relative to formation. The addictive effects may be associated with the exacerbated iron accumulation and osteoclast differentiation.






Similar content being viewed by others
Abbreviations
- ALP:
-
Alkaline phosphatase
- BMD:
-
bone mineral density
- BMMs:
-
Bone marrow macrophages
- Bsp:
-
Bone sialoprotein
- BV/TV:
-
Bone volume fraction
- Col1:
-
Collagen 1
- Ctsk:
-
Cathepsin K
- Dmp1:
-
Dentin matrix acidic phosphoprotein 1
- FAC:
-
Ferric ammonium citrate
- Gapdh:
-
Glyceraldehyde-3-phosphate dehydrogenase
- Hamp:
-
Hepcidin antimicrobial peptide
- IR:
-
Ionizing radiation
- MAR:
-
Mineral apposition rate
- M-CSF:
-
Macrophage colony stimulating factor
- Mmp9:
-
Matrix metallopeptidase 9
- Opn :
-
Osteopontin
- RANKL:
-
Receptor activator for nuclear factor-κB ligand
- Runx2:
-
Runt-related transcription factor 2
- Tb.Th.:
-
Trabecular thickness
- Th.Sp.:
-
Trabecular separation
- Tb.N.:
-
Trabecular number
- TBI:
-
Total body irradiation
- TRAP:
-
Tartrate-resistant acid phosphatase
- V-ATPase:
-
Vacuolar-type H (+)-ATPase
References
Abbaspour N, Hurrell R, Kelishadi R (2014) Review on iron and its importance for human health. J Res Med Sci 19(2):164–174
Balogh E, Paragh G, Jeney V (2018) Influence of iron on bone homeostasis. Pharmaceuticals 11(4):107
Kocpinar EF, Gonul Baltaci N, Ceylan H, Kalin SN, Erdogan O, Budak H (2019) Effect of a prolonged dietary iron intake on the gene expression and activity of the testicular antioxidant defense system in rats. Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01817-0
Budak H, Gonul N, Ceylan H et al (2014) Impact of long term Fe(3)(+) toxicity on expression of glutathione system in rat liver. Environ Toxicol Pharmacol 37(1):365–370
Balogh E, Paragh G, Jeney V (2018) Influence of iron on bone homeostasis. Pharmaceuticals (Basel) 11(4):E107
Mitchell F (2012) High body iron stores lead to bone loss. Nat Rev Endocrinol 8(9):506
Ishii KA, Fumoto T, Iwai K et al (2009) Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15(3):259–266
Jeney V (2017) Clinical impact and cellular mechanisms of iron overload-associated bone loss. Front Pharmacol 8:77
Kim BJ, Ahn SH, Bae SJ et al (2012) Iron overload accelerates bone loss in healthy postmenopausal women and middle-aged men: a 3-year retrospective longitudinal study. J Bone Miner Res 27(11):2279–2290
Zhang J, Qiu X, Xi K et al (2018) Therapeutic ionizing radiation induced bone loss: a review of in vivo and in vitro findings. Connect Tissue Res 59(6):509–522
Zhang J, Wang Z, Wu A et al (2017) Differences in responses to X-ray exposure between osteoclast and osteoblast cells. J Radiat Res 58(6):791–802
Morgan JL, Ritchie LE, Crucian BE (2014) Increased dietary iron and radiation in rats promote oxidative stress, induce localized and systemic immune system responses, and alter colon mucosal environment. FASEB J 28(3):1486–1498
Nelson JM, Stevens RG (1992) Ferritin-iron increases killing of Chinese hamster ovary cells by X-irradiation. Cell Prolif 25(6):579–585
Theriot CA, Westby CM, Morgan JLL et al (2016) High dietary iron increases oxidative stress and radiosensitivity in the rat retina and vasculature after exposure to fractionated gamma radiation. NPJ Microgravity 2:16014
Xiao W, Beibei F, Guangsi S et al (2015) Iron overload increases osteoclastogenesis and aggravates the effects of ovariectomy on bone mass. J Endocrinol 226(3):121–134
Zhang J, Zheng L, Wang Z et al (2019) Lowering iron level protects against bone loss in focally irradiated and contralateral femurs through distinct mechanisms. Bone 120:50–60
Zhang J, Meng X, Ding C et al (2018) Effects of static magnetic fields on bone microstructure and mechanical properties in mice. Electromagn Biol Med 37(2):76–83
Zhang J, Meng X, Ding C et al (2017) Regulation of osteoclast differentiation by static magnetic fields. Electromagn Biol Med 36(1):8–19
Zhang J, Ding C, Shang P (2014) Alterations of mineral elements in osteoblast during differentiation under hypo, moderate and high static magnetic fields. Biol Trace Elem Res 162(1-3):153–157
Girelli D, Nemeth E, Swinkels DW (2016) Hepcidin in the diagnosis of iron disorders. Blood 127(23):2809–2813
Yoshiyama M, Okamoto Y, Izumi S et al (2019) Graphite furnace atomic absorption spectrometric evaluation of iron excretion in mouse urine caused by whole-body gamma irradiation. Biol Trace Elem Res 191(1):149–158
Xu Z, Sun W, Li Y et al (2017) The regulation of iron metabolism by hepcidin contributes to unloading-induced bone loss. Bone 94:152–161
Gehrke SG, Herrmann T, Kulaksiz H et al (2005) Iron stores modulate hepatic hepcidin expression by an HFE-independent pathway. Digestion 72(1):25–32
Ono T, Nakashima T (2018) Recent advances in osteoclast biology. Histochem Cell Biol 149(4):325–341
Chen M, Huang Q, Xu W et al (2014) Low-dose X-ray irradiation promotes osteoblast proliferation, differentiation and fracture healing. PLoS One 9(8):e104016
Szymczyk KH, Shapiro IM, Adams CS (2004) Ionizing radiation sensitizes bone cells to apoptosis. Bone 34(1):148–156
Zhang J, Hu W, Ding C et al (2019) Deferoxamine inhibits iron-uptake stimulated osteoclast differentiation by suppressing electron transport chain and MAPKs signaling. Toxicol Lett 313:50–59
Keenawinna L, Oest ME, Mann KA et al (2013) Zoledronic acid prevents loss of trabecular bone after focal irradiation in mice. Radiat Res 180(1):89–99
Willey JS, Livingston EW, Robbins ME et al (2010) Risedronate prevents early radiation-induced osteoporosis in mice at multiple skeletal locations. Bone 46(1):101–111
Schreurs AS, Shirazi-Fard Y, Shahnazari M et al (2016) Dried plum diet protects from bone loss caused by ionizing radiation. Sci Rep 6:21343
Funding
This study was funded by the National Natural Science Foundation of China (31600674), the Science and Technology Fund of Guizhou Health Commission (gzwjkj2019-1-226), and doctoral funds of Guizhou University of Traditional Chinese Medicine ([2019]44).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of Guizhou University of Traditional Chinese Medicine at which the studies were conducted (Animal Ethics and Welfare Committee at Guizhou University of Traditional Chinese Medicine; Permit No. GZY20190005).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Qiao, P., Yao, G. et al. Ionizing Radiation Exacerbates the Bone Loss Induced by Iron Overload in Mice. Biol Trace Elem Res 196, 502–511 (2020). https://doi.org/10.1007/s12011-019-01929-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01929-7