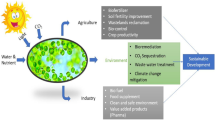
Abstract
Microalgae cultivation, when compared to the growth of higher plants, presents many advantages such as faster growth, higher biomass productivity, and smaller land area requirement for cultivation. For this reason, microalgae are an alternative platform for carotenoid production when compared to the traditional sources. Currently, commercial microalgae production is not well developed but, fortunately, there are several studies aiming to make the large-scale production feasible by, for example, employing different cultivation systems. This review focuses on the main carotenoids from microalgae, comparing them to the traditional sources, as well as a critical analysis about different microalgae cultivation regimes that are currently available and applicable for carotenoid accumulation. Throughout this review paper, we present relevant information about the main commercial microalgae carotenoid producers; the comparison between carotenoid content from food, vegetables, fruits, and microalgae; and the great importance and impact of these molecule applications, such as in food (nutraceuticals and functional foods), cosmetics and pharmaceutical industries, feed (colorants and additives), and healthcare area. Lastly, the different operating systems applied to these photosynthetic cultivations are critically discussed, and conclusions and perspectives are made concerning the best operating system for acquiring high cell densities and, consequently, high carotenoid accumulation.




Similar content being viewed by others
References
Xu, L., Weathers, P. J., Xiong, X. R., & Liu, C. Z. (2009). Microalgal bioreactors: challenges and opportunities. Engineering in Life Sciences, 9(3), 178–189. https://doi.org/10.1002/elsc.200800111.
Walker, T. L., Purton, S., Becker, D. K., & Collet, C. (2005). Microalgae as bioreactors. Plant Cell Reports, 24(11), 629–641. https://doi.org/10.1007/s00299-005-0004-6.
Gouveia, L., & Oliveira, A. C. (2009). Microalgae as a raw material for biofuels production. Journal of Industrial Microbiology and Biotechnology, 36(2), 269–274. https://doi.org/10.1007/s10295-008-0495-6.
Gupta, P. L., Lee, S. M., & Choi, H. J. (2015). A mini review: Photobioreactors for large scale algal cultivation. World Journal of Microbiology and Biotechnology, 31(9), 1409–1417. https://doi.org/10.1007/s11274-015-1892-4.
Chiu, H. F., Liao, J. Y., Lu, Y. Y., Han, Y. C., Shen, Y. C., Venkatakrishnan, K., Golovinskaia, O., & Wang, C. K. (2017). Anti-proliferative, anti-inflammatory and pro-apoptotic effects of Dunaliella salina on human KB oral carcinoma cells. Journal of Food Biochemistry, 41(3), 1–8. https://doi.org/10.1111/jfbc.12349.
Fimbres-Olivarria, D., Carvajal-Millan, E., Lopez-Elias, J. A., Martinez-Robinson, K. G., Miranda-Baeza, A., Martinez-Cordova, L. R., Enriquez-Ocaña, F., & Valdez-Holguin, J. E. (2017). Chemical characterization and antioxidant activity of sulfated polysaccharides from Navicula sp. Food Hydrocolloids, 75, 229–236. https://doi.org/10.1016/j.foodhyd.2017.08.002.
Rosenberg, J. N., Oyler, G. A., Wilkinson, L., & Betenbaugh, M. J. (2008). A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Current Opinion in Biotechnology, 19(5), 430–436. https://doi.org/10.1016/j.copbio.2008.07.008.
Gantar, M., & Svirčev, Z. (2008). Microalgae and cyanobacteria: food for thought. Journal of Phycology, 44(2), 260–268. https://doi.org/10.1111/j.1529-8817.2008.00469.x.
Berman, J., Zorrilla-López, U., Farré, G., Zhu, C., Sandmann, G., Twyman, R. M., Capell, T., & Christou, P. (2015). Nutritionally important carotenoids as consumer products. Phytochemistry Reviews, 14(5), 727–743. https://doi.org/10.1007/s11101-014-9373-1.
Singh, R. N., & Sharma, S. (2012). Development of suitable photobioreactor for algae production—a review. Renewable and Sustainable Energy Reviews, 16(4), 2347–2353. https://doi.org/10.1016/j.rser.2012.01.026.
Del Campo, J. A., García-González, M., & Guerrero, M. G. (2007). Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Applied Microbiology and Biotechnology, 74(6), 1163–1174. https://doi.org/10.1007/s00253-007-0844-9.
Moreno-garcia, L., Adjallé, K., Barnabé, S., & Raghavan, G. S. V. (2017). Microalgae biomass production for a biorefinery system: recent advances and the way towards sustainability. Renewable and Sustainable Energy Reviews, 76, 493–506. https://doi.org/10.1016/j.rser.2017.03.024.
Fernandes, B. D., Mota, A., Teixeira, J. A., & Vicente, A. A. (2015). Continuous cultivation of photosynthetic microorganisms: approaches, applications and future trends. Biotechnology Advances, 33(6), 1228–1245.
Morales-Sánchez, D., Martinez-Rodriguez, O. A., & Martinez, A. (2017). Heterotrophic cultivation of microalgae: production of metabolites of commercial interest. Journal of Chemical Technology and Biotechnology, 92(5), 925–936. https://doi.org/10.1002/jctb.5115.
Carvalho, J. C. M., Bezerra, R. P., Matsudo, M. C., & Sato, S. (2013). Cultivation of Arthrospira (Spirulina) platensis by fed-batch process. In J. W. Lee (Ed.), Advanced biofuels and bioproducts (pp. 781–805). New York: Springer. https://doi.org/10.1007/978-1-4614-3348-4.
Saini, R. K., Nile, S. H., & Park, S. W. (2015). Carotenoids from fruit and vegetables: chemistry, analysis, occurrence, bioavailability and biological activities. Food Research International, 76, 735–750. https://doi.org/10.1016/j.aqpro.2013.07.003.
Shi, X. M., Jiang, Y., & Chen, F. (2002). High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnology Progress, 18(4), 723–727. https://doi.org/10.1021/bp0101987.
Lorenz, R. T., & Cysewski, G. R. (2000). Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends in Biotechnology, 18(4), 160–167.
Gong, M., & Bassi, A. (2016). Carotenoids from microalgae: a review of recent developments. Biotechnology Advances, 34(8), 1396–1412. https://doi.org/10.1016/j.biotechadv.2016.10.005.
Business Communications Company (2015). The global market for carotenoids—code – FOD025E. Retrieved October 23, 2017, from https://www.bccresearch.com/market-research/food-and-beverage/carotenoids-global-market-report-fod025e.html
Rasala, B. A., & Mayfield, S. P. (2015). Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynthesis Research, 123(3), 227–239. https://doi.org/10.1007/s11120-014-9994-7.
Peng, J., Yuan, J. P., Wu, C. F., & Wang, J. H. (2011). Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Marine Drugs, 9(10), 1806–1828. https://doi.org/10.3390/md9101806.
Gammone, M. A., & D’Orazio, N. (2015). Anti-obesity activity of the marine carotenoid fucoxanthin. Marine Drugs, 13(4), 2196–2214. https://doi.org/10.3390/md13042196.
Yaakob, Z., Ali, E., Zainal, A., Mohamad, M., & Takriff, M. S. (2014). An overview: Biomolecules from microalgae for animal feed and aquaculture. Journal of Biological Research (Greece), 21(1), 1–10. https://doi.org/10.1186/2241-5793-21-6.
Grobbelaar, J. U. (2016). AUFWIND: an ambitious German microalgae project for producing third-generation biofuels. South African Journal of Science, 112(9–10), 9–10. https://doi.org/10.17159/sajs.2016/a0174.
Prasanna, R., & Kaushik, B. D. (2010). Evolutionary relationships among cyanobacteria, algae and plants: revisited in the light of Darwinism. In V. P. Sharma (Ed.), Nature at work: ongoing saga of evolution (pp. 119–140). New Delhi: Springer.
Gimpel, J. A., Henríquez, V., & Mayfield, S. P. (2015). In metabolic engineering of eukaryotic microalgae: potential and challenges come with great diversity. Frontiers in Microbiology, 6(DEC), 1–14. https://doi.org/10.3389/fmicb.2015.01376.
Hemaiswarya, S., Raja, R., Ravikumar, R., & Carvalho, I. S. (2013). Microalgae taxonomy and breeding. Biofuel crops: production, physiology and genetics, (January 2016), 44–53. Retrieved from https://www.researchgate.net/publication/286617872
Norton, T. A., Melkonian, M., & Andersen, R. A. (1996). Algal biodiversity*. Phycologia, 35(4), 308–326. https://doi.org/10.2216/i0031-8884-35-4-308.1.
McCann, A. E., & Cullimore, D.. (1979). Residue reviews. In F. A. Gunther (Ed.), Influence of pesticides on soil algal flora (pp. 1–31). Riverside: Springer.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25(3), 294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001.
Boyce, D. G., Lewis, M. R., & Worm, B. (2010). Global phytoplankton decline over the past century. Nature, 466(7306), 591–596. https://doi.org/10.1038/nature09268.
Algaebase. (n.d.). AlgaBase. Retrieved November 17, 2018, from http://www.algaebase.org/
García-Balboa, C., Baselga-Cervera, B., García-Sanchez, A., Igual, J. M., Lopez-Rodas, V., & Costas, E. (2013). Rapid adaptation of microalgae to bodies of water with extreme pollution from uranium mining: an explanation of how mesophilic organisms can rapidly colonise extremely toxic environments. Aquatic Toxicology, 144–145, 116–123. https://doi.org/10.1016/j.aquatox.2013.10.003.
Brock, T. D., Science, S., Series, N., & Feb, N. (2014). Lower pH limit for the existence of blue-green algae: evolutionary and ecological implications., 179(4072), 480–483.
Sakshaug, E., & Slagstad, D. (1991). Light and productivity of phytoplankton in polar marine ecosystems: a physiological view. Polar Research, 10(1), 69–86. https://doi.org/10.1111/j.1751-8369.1991.tb00636.x.
Pushkareva, E., Johansen, J. R., & Elster, J. (2016). A review of the ecology, ecophysiology and biodiversity of microalgae in Arctic soil crusts. Polar Biology, 39(12), 2227–2240. https://doi.org/10.1007/s00300-016-1902-5.
Negro, J. J., & Garrido-Fernández, J. (2000). Astaxanthin is the major carotenoid in tissues of white storks (Ciconia ciconia) feeding on introduced crayfish (Procambarus clarkii). Comparative Biochemistry and Physiology - B Biochemistry and Molecular Biology, 126(3), 347–352. https://doi.org/10.1016/S0305-0491(00)00180-2.
Chandi, G. K., & Gill, B. S. (2011). Production and characterization of microbial carotenoids as an alternative to synthetic colors: a review. International Journal of Food Properties, 14(3), 503–513. https://doi.org/10.1080/10942910903256956.
Bhosale, P., & Bernstein, P. S. (2005). Microbial xanthophylls. Applied Microbiology and Biotechnology, 68(4), 445–455. https://doi.org/10.1007/s00253-005-0032-8.
Eonseon, J., Lee, C.-G., & Polle, J. E. W. (2006). Secondary carotenoid accumulation in Haematococcus Chlorophyceae: biosynthesis, regulation, and biotechnology. Journal of Microbiology and Biotechnology, 16(6), 821–831 Retrieved from http://cat.inist.fr/?aModele=afficheN&cpsidt=17872039.
Ye, Z. W., Jiang, J. G., & Wu, G. H. (2008). Biosynthesis and regulation of carotenoids in Dunaliella: progresses and prospects. Biotechnology Advances, 26(4), 352–360. https://doi.org/10.1016/j.biotechadv.2008.03.004.
Schmidt-Dannert, C. (2000). Engineering novel carotenoids in microorganisms. Current Opinion in Biotechnology, 11(3), 255–261. https://doi.org/10.1016/S0958-1669(00)00093-8.
Grünewald, K., Eckert, M., Hirschberg, J., & Hagen, C. (2000). Phytoene desaturase is localized exclusively in the chloroplast and up-regulated at the mRNA level during accumulation of secondary carotenoids in Haematococcus pluvialis (Volvocales, Chlorophyceae). Plant Physiology, 122(4), 1261–1268. https://doi.org/10.1104/pp.122.4.1261.
Collins, A. M., Jones, H. D. T., Han, D., Hu, Q., Beechem, T. E., & Timlin, J. A. (2011). Carotenoid distribution in living cells of Haematococcus pluvialis (Chlorophyceae). PLoS One, 6(9), 1–7. https://doi.org/10.1371/journal.pone.0024302.
Lemoine, Y., & Schoefs, B. (2010). Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynthesis Research, 106(1–2), 155–177. https://doi.org/10.1007/s11120-010-9583-3.
Ghosh, A., Khanra, S., Mondal, M., Halder, G., Tiwari, O. N., Saini, S., Bhowmick, T. K., & Gayen, K. (2016). Progress toward isolation of strains and genetically engineered strains of microalgae for production of biofuel and other value added chemicals: a review. Energy Conversion and Management, 113, 104–118. https://doi.org/10.1016/j.enconman.2016.01.050.
Rosello Sastre, R., & Posten, C. (2010). The variety of microalgae applications as renewable source. Chemie Ingenieur Technik, 82(11), 1925–1939. https://doi.org/10.1002/cite.201000124.
Borowitzka, M. A. (1992). Algal biotechnology products and processes—matching science and economics. Journal of Applied Phycology, 4(3), 267–279. https://doi.org/10.1007/BF02161212.
Borowitzka, M. A. (2013). High-value products from microalgae—their development and commercialisation. Journal of Applied Phycology, 25(3), 743–756. https://doi.org/10.1007/s10811-013-9983-9.
Deinove (n.d.). Carotenoids market. Natural carotenoids meeting consumer needs. Retrieved November 17, 2018, from http://www.deinove.com/en/profile/strategy-and-markets/carotenoids-market
Li, J., Zhu, D., Niu, J., Shen, S., & Wang, G. (2011). An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnology Advances, 29(6), 568–574. https://doi.org/10.1016/j.biotechadv.2011.04.001.
Nguyen, K. D. (2013). Astaxanthin: a comparative case of synthetic vs. natural production. Chemical and Biomolecular Engineering Publications and Other Works, 1–9. Retrieved from http://trace.tennessee.edu/cgi/viewcontent.cgi?article=1094&context=utk_chembiopubs
Lin, J. H., Lee, D. J., & Chang, J. S. (2015). Lutein production from biomass: marigold flowers versus microalgae. Bioresource Technology, 184, 421–428. https://doi.org/10.1016/j.biortech.2014.09.099.
Grama, B. S., Chader, S., Khelifi, D., Agathos, S. N., & Jeffryes, C. (2014). Induction of canthaxanthin production in a Dactylococcus microalga isolated from the algerian sahara. Bioresource Technology, 151, 297–305. https://doi.org/10.1016/j.biortech.2013.10.073.
Boudreault, G., Cortin, P., Corriveau, L. A., Rousseau, A. P., Tardif, Y., & Malenfant, M. (1983). Canthaxanthine retinopathy: 1. Clinical study in 51 consumers. Canadian Journal of Ophthalmology, 18(7), 325–328.
Hsu, Y. W., Tsai, C. F., Chang, W. H., Ho, Y. C., Chen, W. K., & Lu, F. J. (2008). Protective effects of Dunaliella salina—a carotenoids-rich alga, against carbon tetrachloride-induced hepatotoxicity in mice. Food and Chemical Toxicology, 46(10), 3311–3317. https://doi.org/10.1016/j.fct.2008.07.027.
Hu, C. C., Lin, J. T., Lu, F. J., Chou, F. P., & Yang, D. J. (2008). Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chemistry, 109(2), 439–446. https://doi.org/10.1016/j.foodchem.2007.12.043.
Borowitzka, L. J., Borowitzka, M. A., & Moulton, T. P. (1984). The mass culture of Dunaliella for fine chemicals: from laboratory to pilot plant. Hydrobiologia, 116/117(1), 115–134. https://doi.org/10.1007/BF00027649.
Lamers, P. P., Janssen, M., De Vos, R. C. H., Bino, R. J., & Wijffels, R. H. (2008). Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends in Biotechnology, 26(11), 631–638. https://doi.org/10.1016/j.tibtech.2008.07.002.
Aasen, I. M., Ertesvåg, H., Heggeset, T. M. B., Liu, B., Brautaset, T., Vadstein, O., & Ellingsen, T. E. (2016). Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Applied Microbiology and Biotechnology, 100(10), 4309–4321. https://doi.org/10.1007/s00253-016-7498-4.
Guerin, M., Huntley, M. E., & Olaizola, M. (2003). Haematococcus astaxanthin: applications for human health and nutrition. Trends in Biotechnology, 21(5), 210–216. https://doi.org/10.1016/S0167-7799(03)00078-7.
Kang, C. D., Lee, J. S., Park, T. H., & Sim, S. J. (2005). Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Applied Microbiology and Biotechnology, 68(2), 237–241. https://doi.org/10.1007/s00253-005-1889-2.
Zhang, W., Wang, J., Wang, J., & Liu, T. (2014). Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresource Technology, 158, 329–335. https://doi.org/10.1016/j.biortech.2014.02.044.
Sarada, R., Vidhyavathi, R., Usha, D., & Ravishankar, G. A. (2006). An efficient method for extraction of astaxanthin from green alga Haematococcus pluvialis. Journal of Agricultural and Food Chemistry, 54(20), 7585–7588. https://doi.org/10.1021/jf060737t.
Cardozo, K. H. M., Guaratini, T., Barros, M. P., Falcão, V. R., Tonon, A. P., Lopes, N. P., Campos, S., Torres, M. A., Souza, A. O., Colepicolo, P., & Pinto, E. (2007). Metabolites from algae with economical impact. Comparative Biochemistry and Physiology - C Toxicology and Pharmacology, 146(1–2), 60–78. https://doi.org/10.1016/j.cbpc.2006.05.007.
Zhang, X., Pan, L., Wei, X., Gao, H., & Liu, J. (2007). Impact of astaxanthin-enriched algal powder of Haematococcus pluvialis on memory improvement in BALB/c mice. Environmental Geochemistry and Health, 29(6), 483–489. https://doi.org/10.1007/s10653-007-9117-x.
Shi, X.-M., & Chen, F. (2002). High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnology Progress, 18(4), 723–727. https://doi.org/10.1021/bp0101987.
Basu, H. N., Del Vecchio, A. J., Flider, F., & Orthoefer, F. T. (2001). Nutritional and potential disease prevention properties of carotenoids. JAOCS, Journal of the American Oil Chemists’ Society, 78(7), 665–675. https://doi.org/10.1007/s11746-001-0324-x.
Del Campo, J. A., Rodríguez, H., Moreno, J., Vargas, M. Á., Rivas, J., & Guerrero, M. G. (2001). Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. Journal of Biotechnology, 85(3), 289–295. https://doi.org/10.1016/S0168-1656(00)00380-1.
Sun, Z., Li, T., Zhou, Z., & Jiang, Y. (2015). Microalgae as a source of lutein: chemistry, biosynthesis, and carotenogenesis. Adv Biochem Eng Biotechnol, 153, 37–58. https://doi.org/10.1007/10_2015_331.
Chagas, A. L., Rios, A. O., Jarenkow, A., Marcílio, N. R., Ayub, M. A. Z., & Rech, R. (2015). Production of carotenoids and lipids by Dunaliella tertiolecta using CO2 from beer fermentation. Process Biochemistry, 50(6), 981–988. https://doi.org/10.1016/j.procbio.2015.03.012.
Abe, K., Hattori, H., & Hirano, M. (2007). Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chemistry, 100(2), 656–661. https://doi.org/10.1016/j.foodchem.2005.10.026.
Beatty, S., Boulton, M., Henson, D., Koh, H. H., & Murray, I. J. (1999). Macular pigment and age related macular degeneration. The British Journal of Ophthalmology, 83(7), 867–877. https://doi.org/10.1136/bjo.83.7.867.
Koo, S. Y., Cha, K. H., Song, D. G., Chung, D., & Pan, C. H. (2012). Optimization of pressurized liquid extraction of zeaxanthin from Chlorella ellipsoidea. Journal of Applied Phycology, 24(4), 725–730. https://doi.org/10.1007/s10811-011-9691-2.
Ferruzzi, M. G., & Blakeslee, J. (2007). Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutrition Research, 27(1), 1–12. https://doi.org/10.1016/j.nutres.2006.12.003.
Kim, M., Ahn, J., Jeon, H., Jin, E. S., Cutignano, A., & Romano, G. (2017). Development of a Dunaliella tertiolecta strain with increased zeaxanthin content using random mutagenesis. Marine Drugs, 15(6). https://doi.org/10.3390/md15060189.
Jin, E. S., Feth, B., & Melis, A. (2003). A mutant of the green alga Dunaliella salina constitutively accumulates zeaxanthin under all growth conditions. Biotechnology and Bioengineering, 81(1), 115–124. https://doi.org/10.1002/bit.10459.
Bone, R. A., Landrum, J. T., Guerra, L. H., & Ruiz, C. A. (2003). Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. The Journal of Nutrition, 133(4), 992–998 Retrieved from http://jn.nutrition.org/content/133/4/992.full.pdf.
Schubert, N., García-Mendoza, E., & Pacheco-Ruiz, I. (2006). Carotenoid composition of marine red algae. Journal of Phycology, 42(6), 1208–1216. https://doi.org/10.1111/j.1529-8817.2006.00274.x.
Mishra, N., & Mishra, N. (2018). Exploring the biologically active metabolites of Isochrysis galbana in pharmaceutical interest: an overview. International Journal of Pharmaceutical Sciences and Research, 9(6), 2162–2174. https://doi.org/10.13040/IJPSR.0975-8232.9(6).2162-74.
Kim, S. M., Kang, S. W., Kwon, O. N., Chung, D., & Pan, C. H. (2012). Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: characterization of extraction for commercial application. Journal of the Korean Society for Applied Biological Chemistry, 55(4), 477–483. https://doi.org/10.1007/s13765-012-2108-3.
Lu, X., Sun, H., Zhao, W., Cheng, K., Chen, F., & Liu, B. (2018). A hetero-photoautotrophic two-stage cultivation process for production of fucoxanthin by the marine diatom Nitzschia laevis. Marine Drugs, 16(7), 219. https://doi.org/10.3390/md16070219.
Ishika, T., Moheimani, N. R., Bahri, P. A., Laird, D. W., Blair, S., & Parlevliet, D. (2017). Halo-adapted microalgae for fucoxanthin production: effect of incremental increase in salinity. Algal Research, 28, 66–73. https://doi.org/10.1016/j.algal.2017.10.002.
Molina-Miras, A., López-rosales, L., Sánchez-mirón, A., & Cerón-garcía, M. C. (2018). Long-term culture of the marine dinoflagellate microalga Amphidinium carterae in an indoor LED-lighted raceway photobioreactor: production of carotenoids and fatty acids. Bioresource Technology, 265, 257–267.
Assunção, J., Catarina Guedes, A., & Xavier Malcata, F. (2017). Biotechnological and pharmacological applications of biotoxins and other bioactive molecules from dinoflagellates. Marine Drugs, 15(12). https://doi.org/10.3390/md15120393.
Sugawara, T., Yamashita, K., Sakai, S., Asai, A., Nagao, A., Shiraishi, T., Imai, I., & Hirata, T. (2007). Induction of apoptosis in DLD-1 human colon cancer cells by peridinin isolated from the dinoflagellate, Heterocapsa triquetra. Bioscience, Biotechnology, and Biochemistry, 71(4), 1069–1072. https://doi.org/10.1271/bbb.60597.
Benstein, R. M., Çebi, Z., Podola, B., & Melkonian, M. (2014). Immobilized growth of the peridinin-producing marine dinoflagellate Symbiodinium in a simple biofilm photobioreactor. Marine Biotechnology, 16(6), 621–628. https://doi.org/10.1007/s10126-014-9581-0.
Ishikawa, C., Jomori, T., Tanaka, J., Senba, M., & Mori, N. (2016). Peridinin, a carotenoid, inhibits proliferation and survival of HTLV-1-infected T-cell lines. International Journal of Oncology, 49(4), 1713–1721. https://doi.org/10.3892/ijo.2016.3648.
Cyanotech (n.d.). BioAstin® Hawaiian Astaxanthin®. Retrieved November 17, 2018, from https://www.cyanotech.com/astaxanthin/
Algatechnologies (n.d.). Our microalgae. Retrieved November 17, 2018, from https://www.algatech.com/
Nature Beta Technologies. (n.d.). Retrieved November 17, 2018, from http://wondercare.co.in/nature/nature.html
AquaCarotene (2014). Plankton Australia Pty Limited. Retrieved November 17, 2018, from http://www.planktonaustralia.com/
Esatbeyoglu, T., & Rimbach, G. (2017). Canthaxanthin: from molecule to function. Molecular Nutrition and Food Research, 61(6), 1–17. https://doi.org/10.1002/mnfr.201600469.
Sommerburg, O., Keunen, J. E., Bird, A. C., & van Kuijk, F. J. (1998). Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. The British Journal of Ophthalmology, 82(8), 907–910. https://doi.org/10.1136/BJO.82.8.907.
Piccaglia, R., Marotti, M., & Grandi, S. (1998). Lutein and lutein ester content in different types of Tagetes patula and T. erecta. Industrial Crops and Products, 8(1), 45–51. https://doi.org/10.1016/S0926-6690(97)10005-X.
Holden, J. M., Eldridge, A. L., Beecher, G. R., Marilyn Buzzard, I., Bhagwat, S., Davis, C. S., Douglass, L. W., Gebhardt, S., Haytowitz, D., & Schakel, S. (1999). Carotenoid content of U.S. foods: an update of the database. Journal of Food Composition and Analysis, 12(3), 169–196. https://doi.org/10.1006/jfca.1999.0827.
Kopsell, D. A., Lefsrud, M. G., Kopsell, D. E., Wenzel, A. J., Gerweck, C., & Curran-Celentano, J. (2006). Spinach cultigen variation for tissue carotenoid concentrations influences human serum carotenoid levels and macular pigment optical density following a 12-week dietary intervention. Journal of Agricultural and Food Chemistry, 54(21), 7998–8005. https://doi.org/10.1021/jf0614802.
Lu, Q. Y., Arteaga, J. R., Zhang, Q., Huerta, S., Go, V. L. W., & Heber, D. (2005). Inhibition of prostate cancer cell growth by an avocado extract: role of lipid-soluble bioactive substances. Journal of Nutritional Biochemistry, 16(1), 23–30. https://doi.org/10.1016/j.jnutbio.2004.08.003.
Nishiyama, I., Fukuda, T., & Oota, T. (2007). Cultivar difference in chlorophyll, lutein and β-carotene content in the fruit of kiwifruit and other actinidia species. Acta Horticulturae, 753, 473–478.
Moros, E. E., Darnoko, D., Cheryan, M., Perkins, E. G., & Jerrell, J. (2002). Analysis of xanthophylls in corn by HPLC. Journal of Agricultural and Food Chemistry, 50(21), 5787–5790. https://doi.org/10.1021/jf020109l.
Perry, A., Rasmussen, H., & Johnson, E. J. (2009). Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. Journal of Food Composition and Analysis, 22(1), 9–15. https://doi.org/10.1016/j.jfca.2008.07.006.
Sajilata, M. G., Singhal, R. S., & Kamat, M. Y. (2008). The carotenoid pigment zeaxanthin—a review. Comprehensive Reviews in Food Science and Food Safety, 7(1), 29–49. https://doi.org/10.1111/j.1541-4337.2007.00028.x.
Seddon, J., Ajani, U., & Sperduto, R. (1994). Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. J. Am. J. Med. Assoc., 272(18), 1413–1420. Retrieved from http://archpsyc.jamanetwork.com/article.aspx?articleid=382145.
Lin, T. M., Durance, T. D., & Scaman, C. H. (1998). Characterization of vacuum microwave, air and freeze dried carrot slices. Food Research International, 31(2), 111–117. https://doi.org/10.1016/S0963-9969(98)00070-2.
Favacho, F. S., Lima, J. S. S., Neto, F. B., Silva, J. N., & Barros, A. P. (2017). Productive and economic efficiency of carrot intercropped with cowpea vegetable resulting from green manure and different spatial arrangements. Revista Ciencia Agronomica, 48(2), 337–346. https://doi.org/10.5935/1806-6690.20170039.
Frusciante, L., Carli, P., Ercolano, M. R., Pernice, R., Di Matteo, A., Fogliano, V., & Pellegrini, N. (2007). Antioxidant nutritional quality of tomato. Molecular Nutrition and Food Research, 51(5), 609–617. https://doi.org/10.1002/mnfr.200600158.
Shi, J., & Le Maguer, M. (2000). Lycopene in tomatoes: chemical and physical properties affected by food processing. Critical Reviews in Food Science and Nutrition, 40(1), 1–42. https://doi.org/10.1080/10408690091189275.
Renju, G. L., Muraleedhara Kurup, G., & Bandugula, V. R. (2014). Effect of lycopene isolated from Chlorella marina on proliferation and apoptosis in human prostate cancer cell line PC-3. Tumour Biology: the journal of the International Society for Oncodevelopmental Biology and Medicine, 35(11), 10747–10758. https://doi.org/10.1007/s13277-014-2339-5.
Renju, G. L., Kurup, G. M., & Kumari, C. H. S. (2013). Anti-inflammatory activity of lycopene isolated from Chlorella marina on type II collagen induced arthritis in Sprague Dawley rats. Immunopharmacology and Immunotoxicology, 35(2), 282–291. https://doi.org/10.3109/08923973.2012.742534.
Saini, R. K., & Keum, Y.-S. (2018). Carotenoid extraction methods: a review of recent developments. Food Chemistry, 240, 90–103. https://doi.org/10.1016/j.foodchem.2017.07.099.
Edge, R., McGarvey, D. J., & Truscott, T. G. (1997). The carotenoids as anti-oxidants—a review. Journal of Photochemistry and Photobiology. B, Biology, 41(3), 189–200. https://doi.org/10.1016/S1011-1344(97)00092-4.
Chuang, W. C., Ho, Y. C., Liao, J. W., & Lu, F. J. (2014). Dunaliella salina exhibits an antileukemic immunity in a mouse model of WEHI-3 leukemia cells. Journal of Agricultural and Food Chemistry, 62(47), 11479–11487. https://doi.org/10.1021/jf503564b.
Jayappriyan, K. R., Rajkumar, R., Venkatakrishnan, V., Nagaraj, S., & Rengasamy, R. (2013). In vitro anticancer activity of natural β-carotene from Dunaliella salina EU5891199 in PC-3 cells. Biomedicine & Preventive Nutrition, 3(2), 99–105. https://doi.org/10.1016/j.bionut.2012.08.003.
Sheu, M.-J., Huang, G.-J., Wu, C.-H., Chen, J.-S., Chang, H.-Y., Chang, S.-J., & Chung, J.-G. (2008). Ethanol extract of Dunaliella salina induces cell cycle arrest and apoptosis in A549 human non-small cell lung cancer cells. In Vivo, 22(3), 369–378.
Grung, M., D’Souza, F. M. L., Borowitzka, M., & Liaaen-Jensen, S. (1992). Algal carotenoids 51. Secondary carotenoids 2. Haematococcus pluvialis aplanospores as a source of (3S, 3′S)-astaxanthin esters. Journal of Applied Phycology, 4(2), 165–171. https://doi.org/10.1007/BF02442465.
Rao, A. R., Sindhuja, H. N., Dharmesh, S. M., Sankar, K. U., Sarada, R., & Ravishankar, G. A. (2013). Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. Journal of Agricultural and Food Chemistry, 61(16), 3842–3851. https://doi.org/10.1021/jf304609j.
Otton, R., Marin, D. P., Bolin, A. P., dos Santos, R. d. C. M., Polotow, T. G., Sampaio, S. C., & de Barros, M. P. (2010). Astaxanthin ameliorates the redox imbalance in lymphocytes of experimental diabetic rats. Chemico-Biological Interactions, 186(3), 306–315. https://doi.org/10.1016/j.cbi.2010.05.011.
Monroy-Ruiz, J., Sevilla, M.-Á., Carrón, R., & Montero, M.-J. (2011). Astaxanthin-enriched-diet reduces blood pressure and improves cardiovascular parameters in spontaneously hypertensive rats. Pharmacological Research: the official journal of the Italian Pharmacological Society, 63(1), 44–50. https://doi.org/10.1016/j.phrs.2010.09.003.
Kläui, H. (1982). Industrial and commercial uses of carotenoids. Carotenoid chemistry and biochemistry. International Union of Pure and Applied Chemistry. https://doi.org/10.1016/B978-0-08-026224-6.50026-7.
Dwyer, J. H., Navab, M., Dwyer, K. M., Hassan, K., Sun, P., Shircore, A., Hama-Levy, S., Hough, G., Wang, X., Drake, T., Merz, C. N. B., & Fogelman, A. M. (2001). Oxygenated carotenoid lutein and progression of early atherosclerosis: the Los Angeles Atherosclerosis Study. Circulation, 103(24), 2922–2927. https://doi.org/10.1161/01.CIR.103.24.2922.
Le Marchand, L., Hankin, J. H., Kolonel, L. N., Beecher, G. R., Wilkens, L. R., & Zhao, L. P. (1993). Intake of specific carotenoids and lung cancer risk. Cancer Epidemiology, Biomarkers & Prevention, 2, 183–187.
Ziegler, R. G., Elizabeth, A., Hartge, P., Mcadams, J., Janet, B., Mason, T. J., & Fraumeni, J. F. (1996). The importance of alpha-carotene, beta-carotene, and other phytochemicals in the etiology of lung cancer. Journal of the National Cancer Institute, 88(9).
Chiu, C. J., & Taylor, A. (2007). Nutritional antioxidants and age-related cataract and maculopathy. Experimental Eye Research, 84(2), 229–245. https://doi.org/10.1016/j.exer.2006.05.015.
Schnebelen-Berthier, C., Acar, N., Pouillart, P., Thabuis, C., Rodriguez, B., Depeint, F., Clerc, E., Mathiaud, A., Bourdillon, A., Baert, B., Bretillon, L., & Lecerf, J.-M. (2015). Incorporation of lutein and docosahexaenoic acid from dietary microalgae into the retina in quail. International Journal of Food Sciences and Nutrition, 66(2), 222–229. https://doi.org/10.3109/09637486.2014.971227.
Olmedilla, B., Granado, F., Blanco, I., Vaquero, M., & Cajigal, C. (2001). Lutein in patients with cataracts and age-related macular degeneration: a long-term supplementation study. Journal of the Science of Food and Agriculture, 81(9), 904–909. https://doi.org/10.1002/jsfa.905.
Wei, D., Chen, F., Chen, G., Zhang, X., Liu, L., & Zhang, H. (2008). Enhanced production of lutein in heterotrophic Chlorella protothecoides by oxidative stress. Science in China, Series C: Life Sciences, 51(12), 1088–1093. https://doi.org/10.1007/s11427-008-0145-2.
Suhonen, R., & Plosila, M. (1981). The effect of beta-carotene in combination with canthaxanthin, Ro 8-8427 (Phenoro), in treatment of polymorphous light eruptions., 163, 172–176.
Gensler, H. L., & Holladay, K. (1990). Enhanced resistance to an antigenic tumor in immunosuppressed mice by dietary retinyl palmitate canthaxanthin. Cancer Letter, 49, 231–236.
Ernst, H. (2002). Recent advances in industrial carotenoid synthesis*. Pure and Applied Chemistry, 74(11), 2213–2226.
Malis, S. A., Cohen, E., & Ben Amotz, A. (1993). Accumulation of canthaxanthin in Chlorella emersonii. Physiologia Plantarum, 87(2), 232–236. https://doi.org/10.1111/j.1399-3054.1993.tb00148.x.
Hanagata, N., & Dubinsky, Z. (1999). Secondary carotenoid accumulation in Scenedesmus komarekii (Chlorophycea, Chlorophyta). Journal of Phycology, 35(5), 960–966. https://doi.org/10.1046/j.1529-8817.1999.3550960.x.
Pelah, D., Sintov, A., & Cohen, E. (2004). The effect of salt stress on the production of canthaxanthin and astaxanthin by Chlorella zofingiensis grown under limited light intensity. World Journal of Microbiology and Biotechnology, 20(5), 483–486. https://doi.org/10.1023/B:WIBI.0000040398.93103.21.
Bar, E., Rise, M., Vishkautsan, M., & Arad, S. M. (1995). Pigment and structural changes in Chlorella zofingiensis upon light and nitrogen stress. Journal of Plant Physiology, 146(4), 527–534. https://doi.org/10.1016/S0176-1617(11)82019-5.
Liu, J., Sun, Z., Gerken, H., Liu, Z., Jiang, Y., & Chen, F. (2014). Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: biology and industrial potential. Marine Drugs, 12(6), 3487–3515. https://doi.org/10.3390/md12063487.
Chen, J. h., Liu, L., & Wei, D. (2017). Enhanced production of astaxanthin by Chromochloris zofingiensis in a microplate-based culture system under high light irradiation. Bioresource Technology, 245, 518–529. https://doi.org/10.1016/j.biortech.2017.08.102.
Jansen, R. J., Robinson, D. P., Stolzenberg-solomon, R. Z., William, R., De Andrade, M., Oberg, A. L., Rabe, K. G., Anderson, K. E., Olson, J. E., Sinha, R., & Petersen, G. M. (2014). Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. Journal of Gastrointestinal Cancer, 44(2), 152–161. https://doi.org/10.1007/s12029-012-9441-y.Nutrients.
Chew, E. Y., Clemons, T. E., SanGiovanni, J. P., Danis, R., Ferris, F. L., III, Elman, M., et al. (2013). Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration. Journal of the American Medical Association, 309(19), 2005–2015. https://doi.org/10.1001/jama.2013.4997.
Trumbo, P. R., & Ellwood, K. C. (2018). Lutein and zeaxanthin intakes and risk of age-related macular degeneration and cataracts: an evaluation using the Food and Drug Administration’s evidence-based review system for health claims 1–3. The American Journal of Clinical Nutrition, 84, 971–974.
Bone, R. A., Landrum, J. T., Hime, G. W., & Cains, A. (1993). Stereochemistry of the human macular carotenoids. Investigative Ophthalmology & Visual Science, 34(6), 2033–2040.
Bernstein, P. S., Li, B., Vachali, P. P., Gorusupudi, A., Shyam, R., Henriksen, B. S., & Nolan, J. M. (2015). Progress in retinal and eye research lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Progress in Retinal and Eye Research, 50, 34–66. https://doi.org/10.1016/j.preteyeres.2015.10.003.
Maccarrone, M., Bari, M., Gasperi, V., & Demmig-Adams, B. (2005). The photoreceptor protector zeaxanthin induces cell death in neuroblastoma cells. Anticancer Research, 25(6B), 3871–3876.
Kwang, H. C., Song, Y. I. K., & Lee, D. U. (2008). Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. Journal of Agricultural and Food Chemistry, 56(22), 10521–10526. https://doi.org/10.1021/jf802111x.
Othman, R., Mohd Zaifuddin, F. A., & Hassan, N. M. (2014). Carotenoid biosynthesis regulatory mechanisms in plants. Journal of Oleo Science, 63(8), 753–760. https://doi.org/10.5650/jos.ess13183.
Rebolloso Fuentes, M. M., Acién Fernández, G. G., Sánchez Pérez, J. A., & Guil Guerrero, J. L. (2000). Biomass nutrient profiles of the microalga Porphyridium cruentum. Food Chemistry, 70(3), 345–353. https://doi.org/10.1016/S0308-8146(00)00101-1.
Juin, C., Oliveira Junior, R. G. de, Fleury, A., Oudinet, C., Pytowski, L., Bérard, J. B., Nicolau, E., Thiéry, V., Lanneluc, I., Beaugeard, L., Prunier, G., Da Silva Almeida, J. R.G, Picot, L. (2018). Zeaxanthin from Porphyridium purpureum induces apoptosis in human melanoma cells expressing the oncogenic BRAF V600E mutation and sensitizes them to the BRAF inhibitor vemurafenib. Brazilian Journal of Pharmacognosy, 28(4), 457–467. doi:https://doi.org/10.1016/j.bjp.2018.05.009.
Barton-Duell, P. (1995). The role of dietary antioxidants in prevention of artherosclerosis. Endocrinologist, 5(5), 347–356.
Giovannucci, E. (1999). Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. Journal of the National Cancer Institute, 91(4), 317–331. https://doi.org/10.1093/jnci/91.4.317.
Wolf, F., Nonomura, A., & Bassham, J. (1985). Growth and branched hydrocarbon production in a strain of Botryococcus braunii Chlorophyta. Journal of Phycology, 21, 388–396.
Metzger, P., & Largeau, C. (2005). Botryococcus braunii: a rich source for hydrocarbons and related ether lipids. Applied Microbiology and Biotechnology, 66(5), 486–496. https://doi.org/10.1007/s00253-004-1779-z.
Bwapwa, J. K., Anandraj, A., & Trois, C. (2017). Possibilities for conversion of microalgae oil into aviation fuel: a review. Renewable and Sustainable Energy Reviews, 80, 1345–1354. https://doi.org/10.1016/j.rser.2017.05.224.
Matsuura, H., Watanabe, M. M., & Kaya, K. (2012). Echinenone production of a dark red-coloured strain of Botryococcus braunii. Journal of Applied Phycology, 24(4), 973–977. https://doi.org/10.1007/s10811-011-9719-7.
Abidov, M., Ramazanov, Z., Seifulla, R., & Grachev, S. (2010). The effects of Xanthigen™ in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes, Obesity and Metabolism, 12(1), 72–81. https://doi.org/10.1111/j.1463-1326.2009.01132.x.
Wang, S., Verma, S. K., Hakeem Said, I., Thomsen, L., Ullrich, M. S., & Kuhnert, N. (2018). Changes in the fucoxanthin production and protein profiles in Cylindrotheca closterium in response to blue light-emitting diode light. Microbial Cell Factories, 17(1), 1–13. https://doi.org/10.1186/s12934-018-0957-0.
Wang, H., Zhang, Y., Chen, L., Cheng, W., & Liu, T. (2018). Combined production of fucoxanthin and EPA from two diatom strains Phaeodactylum tricornutum and Cylindrotheca fusiformis cultures. Bioprocess and Biosystems Engineering, 41(7), 1061–1071. https://doi.org/10.1007/s00449-018-1935-y.
Xia, S., Wang, K., Wan, L., Li, A., Hu, Q., & Zhang, C. (2013). Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Marine Drugs, 11(7), 2667–2681. https://doi.org/10.3390/md11072667.
Ugwu, C. U., Aoyagi, H., & Uchiyama, H. (2008). Photobioreactors for mass cultivation of algae. Bioresource Technology, 99(10), 4021–4028. https://doi.org/10.1016/j.biortech.2007.01.046.
Carvalho, J. C. M., Matsudo, M. C., Bezerra, R. P., Ferreira-Camargo, L. S., & Sato, S. (2014). Microalgae bioreactors. In R. Bajpai, A. Prokop, & M. Zappi (Eds.), Algal biorefineries (pp. 83–126). Netherlands: Springer.
Borowitzka, M. (1999). Commercial production of microalgae: pond, tanks, tubes and fermenters. Journal of Biotechnology, 70(1–3), 313–321.
Brennan, L., & Owende, P. (2010). Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renewable and Sustainable Energy Reviews, 14(2), 557–577. https://doi.org/10.1016/j.rser.2009.10.009.
Ferreira, L. S., Rodrigues, M. S., Converti, A., Sato, S., & Carvalho, J. C. M. (2012). Arthrospira (Spirulina) platensis cultivation in tubular photobioreactor: use of no-cost CO2 from ethanol fermentation. Applied Energy, 92, 379–385. https://doi.org/10.1016/j.apenergy.2011.11.019.
Matsudo, M. C., Bezerra, R. P., Converti, A., Sato, S., & Carvalho, J. C. M. (2011). CO2 from alcoholic fermentation for continuous cultivation of Arthrospira (Spirulina) platensis in tubular photobioreactor using urea as nitrogen source. Biotechnology Progress, 27(3), 650–656. https://doi.org/10.1002/btpr.581.
Pérez-Mora, L. S., Matsudo, M. C., Cezare-Gomes, E. A., & Carvalho, J. C. M. (2016). An investigation into producing Botryococcus braunii in a tubular photobioreactor. Journal of Chemical Technology and Biotechnology, 91(12), 3053–3060. https://doi.org/10.1002/jctb.4934.
Eze, C. N., Ogbonna, J. C., Ogbonna, I. O., & Aoyagi, H. (2017). A novel flat plate air-lift photobioreactor with inclined reflective broth circulation guide for improved biomass and lipid productivity by Desmodesmus subspicatus LC172266. Journal of Applied Phycology, 29(6), 2745–2754. https://doi.org/10.1007/s10811-017-1153-z.
Liao, Q., Sun, Y., Huang, Y., Xia, A., Fu, Q., & Zhu, X. (2017). Simultaneous enhancement of Chlorella vulgaris growth and lipid accumulation through the synergy effect between light and nitrate in a planar waveguide flat-plate photobioreactor. Bioresource Technology, 243, 528–538. https://doi.org/10.1016/j.biortech.2017.06.091.
Gao, B., Chen, A., Zhang, W., Li, A., & Zhang, C. (2017). Co-production of lipids, eicosapentaenoic acid, fucoxanthin, and chrysolaminarin by Phaeodactylum tricornutum cultured in a flat-plate photobioreactor under varying nitrogen conditions. Journal of Ocean University of China, 16(5), 916–924. https://doi.org/10.1007/s11802-017-3174-2.
Koller, A. P., Löwe, H., Schmid, V., Mundt, S., & Weuster-Botz, D. (2017). Model-supported phototrophic growth studies with Scenedesmus obtusiusculus in a flat-plate photobioreactor. Biotechnology and Bioengineering, 114(2), 308–320. https://doi.org/10.1002/bit.26072.
Koller, A. P., Wolf, L., & Weuster-Botz, D. (2017). Reaction engineering analysis of Scenedesmus ovalternus in a flat-plate gas-lift photobioreactor. Bioresource Technology, 225, 165–174. https://doi.org/10.1016/j.biortech.2016.11.025.
Hulatt, C. J., Wijffels, R. H., Bolla, S., & Kiron, V. (2017). Production of fatty acids and protein by Nannochloropsis in flat-plate photobioreactors. PLoS One, 12(1), 1–17. https://doi.org/10.1371/journal.pone.0170440.
López-Rosales, L., García-Camacho, F., Sánchez-Mirón, A., Contreras-Gómez, A., & Molina-Grima, E. (2017). Modeling shear-sensitive dinoflagellate microalgae growth in bubble column photobioreactors. Bioresource Technology, 245(Pt A), 250–257. https://doi.org/10.1016/j.biortech.2017.08.161.
Plouviez, M., Shilton, A., Packer, M. A., & Guieysse, B. (2017). N2O emissions during microalgae outdoor cultivation in 50 L column photobioreactors. Algal Research, 26, 348–353. https://doi.org/10.1016/j.algal.2017.08.008.
Kim, Z. H., Park, Y. S., Ryu, Y. J., & Lee, C. G. (2017). Enhancing biomass and fatty acid productivity of Tetraselmis sp. in bubble column photobioreactors by modifying light quality using light filters. Biotechnology and Bioprocess Engineering, 22(4), 397–404. https://doi.org/10.1007/s12257-017-0200-6.
Huntley, M. E., Johnson, Z. I., Brown, S. L., Sills, D. L., Gerber, L., Archibald, I., Machesky, S. C., Granados, J., Beal, C., & Greene, C. H. (2015). Demonstrated large-scale production of marine microalgae for fuels and feed. Algal Research, 10, 249–265. https://doi.org/10.1016/j.algal.2015.04.016.
Cyanotech Corporation. (2018). Cyanotech—astaxanthin process. Retrieved August 22, 2018, from https://www.cyanotech.com/astaxanthin/astaxanthin-process/
Fu, W., Paglia, G., Magnúsdóttir, M., Steinarsdóttir, E. A., Gudmundsson, S., Palsson, B. Ó., Andrésson, Ó. S., & Brynjólfsson, S. (2014). Effects of abiotic stressors on lutein production in the green microalga Dunaliella salina. Microbial Cell Factories, 13(1), 3. https://doi.org/10.1186/1475-2859-13-3.
Cuaresma, M., Casal, C., Forján, E., & Vílchez, C. (2011). Productivity and selective accumulation of carotenoids of the novel extremophile microalga Chlamydomonas acidophila grown with different carbon sources in batch systems. Journal of Industrial Microbiology and Biotechnology, 38(1), 167–177. https://doi.org/10.1007/s10295-010-0841-3.
Castro-Puyana, M., Pérez-Sánchez, A., Valdés, A., Ibrahim, O. H. M., Suarez-Álvarez, S., Ferragut, J. A., Micol, V., Cifuentes, A., Ibáñez, E., & García-Cañas, V. (2017). Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Research International, 99(Pt 3), 1048–1055. https://doi.org/10.1016/j.foodres.2016.05.021.
Ma, R., Thomas-Hall, S. R., Chua, E. T., Eltanahy, E., Netzel, M. E., Netzel, G., Lu, Y., & Schenk, P. M. (2018). LED power efficiency of biomass, fatty acid, and carotenoid production in Nannochloropsis microalgae. Bioresource Technology, 252(December 2017), 118–126. https://doi.org/10.1016/j.biortech.2017.12.096.
Pan-utai, W., Parakulsuksatid, P., & Phomkaivon, N. (2017). Effect of inducing agents on growth and astaxanthin production in Haematococcus pluvialis: organic and inorganic. Biocatalysis and Agricultural Biotechnology, 12, 152–158. https://doi.org/10.1016/j.bcab.2017.10.004.
Dayananda, C., & Kumudha, A. (2010). Isolation, characterization and outdoor cultivation of green microalgae Botryococcus sp. Scientific Research and Essays, 5(17), 2497–2505 Retrieved from http://www.academicjournals.org/sre/pdf/pdf2010/4Sep/Dayanandae al.pdf.
Zhu, L. (2015). Microalgal culture strategies for biofuel production: a review. Biofuels, Bioproducts and Biorefining, 9(6), 801–814. https://doi.org/10.1002/bbb.1576.
García-cañedo, J. C., Cristiani-urbina, E., Flores-ortiz, C. M., Ponce-noyola, T., Esparza-garcía, F., & Cañizares-villanueva, R. O. (2016). Batch and fed-batch culture of Scenedesmus incrassatulus: effect over biomass, carotenoid profile and concentration, photosynthetic efficiency and non-photochemical quenching. Algal, 13, 41–52. https://doi.org/10.1016/j.algal.2015.11.013.
Sun, N., Wang, Y., Li, Y. T., Huang, J. C., & Chen, F. (2008). Sugar-based growth, astaxanthin accumulation and carotenogenic transcription of heterotrophic Chlorella zofingiensis (Chlorophyta). Process Biochemistry, 43(11), 1288–1292. https://doi.org/10.1016/j.procbio.2008.07.014.
Chen, C. Y., Lu, I. C., Nagarajan, D., Chang, C. H., Ng, I. S., Lee, D. J., & Chang, J. S. (2018). A highly efficient two-stage cultivation strategy for lutein production using heterotrophic culture of Chlorella sorokiniana MB-1-M12. Bioresource Technology, 253, 141–147. https://doi.org/10.1016/j.biortech.2018.01.027.
Park, J. C., Choi, S. P., Hong, M. E., & Sim, S. J. (2014). Enhanced astaxanthin production from microalga, Haematococcus pluvialis by two-stage perfusion culture with stepwise light irradiation. Bioprocess and Biosystems Engineering, 37(10), 2039–2047. https://doi.org/10.1007/s00449-014-1180-y.
Liu, J., Mao, X., Zhou, W., & Guarnieri, M. T. (2016). Simultaneous production of triacylglycerol and high-value carotenoids by the astaxanthin-producing oleaginous green microalga Chlorella zofingiensis. Bioresource Technology, 214, 319–327. https://doi.org/10.1016/j.biortech.2016.04.112.
Dineshkumar, R., Subramanian, G., Dash, S. K., & Sen, R. (2016). Development of an optimal light-feeding strategy coupled with semi-continuous reactor operation for simultaneous improvement of microalgal photosynthetic efficiency, lutein production and CO2 sequestration. Biochemical Engineering Journal, 113, 47–56. https://doi.org/10.1016/j.bej.2016.05.011.
Prieto, A., Pedro Cañavate, J., & García-González, M. (2011). Assessment of carotenoid production by Dunaliella salina in different culture systems and operation regimes. Journal of Biotechnology, 151(2), 180–185. https://doi.org/10.1016/j.jbiotec.2010.11.011.
García-González, M., Moreno, J., Manzano, J. C., Florencio, F. J., & Guerrero, M. G. (2005). Production of Dunaliella salina biomass rich in 9-cis-β-carotene and lutein in a closed tubular photobioreactor. Journal of Biotechnology, 115(1), 81–90. https://doi.org/10.1016/j.jbiotec.2004.07.010.
Chaumont Daniel, C. T. (1995). Carotenoid content in growing cells of Haematococcus pluvialis during a sunlight cycle. Journal of Applied Phycology, 7.6(1995), 529–537 APA, (1993), 529–537.
Barbosa, M. J., Zijffers, J. W., Nisworo, A., Vaes, W., Van Schoonhoven, J., & Wijffels, R. H. (2005). Optimization of biomass, vitamins, and carotenoid yield on light energy in a flat-panel reactor using the A-stat technique. Biotechnology and Bioengineering, 89(2), 233–242. https://doi.org/10.1002/bit.20346.
Cerón, M. C., García-Malea, M. C., Rivas, J., Acien, F. G., Fernandez, J. M., Del Río, E., Guerrero, M. G., & Molina, E. (2007). Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Applied Microbiology and Biotechnology, 74(5), 1112–1119. https://doi.org/10.1007/s00253-006-0743-5.
Wang, S. K., Stiles, A. R., Guo, C., & Liu, C. Z. (2014). Microalgae cultivation in photobioreactors: an overview of light characteristics. Engineering in Life Sciences, 14(6), 550–559. https://doi.org/10.1002/elsc.201300170.
Carvalho, A. P., Silva, S. O., Baptista, J. M., & Malcata, F. X. (2011). Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Applied Microbiology and Biotechnology, 89(5), 1275–1288. https://doi.org/10.1007/s00253-010-3047-8.
Blanken, W., Cuaresma, M., Wijffels, R. H., & Janssen, M. (2013). Cultivation of microalgae on artificial light comes at a cost. Algal Research, 2(4), 333–340. https://doi.org/10.1016/j.algal.2013.09.004.
Matthijs, H. C. P., Balke, H., Van Hes, U. M., Kroon, B. M. A., Mur, L. R., & Binot, R. A. (1996). Application of light-emitting diodes in bioreactors: flashing light effects and energy economy in algal culture (Chlorella pyrenoidosa). Biotechnology and Bioengineering, 50(1), 98–107. https://doi.org/10.1002/(SICI)1097-0290(19960405)50:1<98::AID-BIT11>3.0.CO;2-3.
Schulze, P. S. C., Barreira, L. A., Pereira, H. G. C., Perales, J. A., & Varela, J. C. S. (2014). Light emitting diodes (LEDs) applied to microalgal production. Trends in Biotechnology, 32(8), 422–430. https://doi.org/10.1016/j.tibtech.2014.06.001.
Kula, M., Rys, M., Mozdzeń, K., & Skoczowski, A. (2014). Metabolic activity, the chemical composition of biomass and photosynthetic activity of Chlorella vulgaris under different light spectra in photobioreactors. Engineering in Life Sciences, 14(1), 57–67. https://doi.org/10.1002/elsc.201200184.
Fu, W., Guomundsson, Ó., Paglia, G., Herjólfsson, G., Andrésson, Ó. S., Palsson, B. O., & Brynjólfsson, S. (2013). Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Applied Microbiology and Biotechnology, 97(6), 2395–2403. https://doi.org/10.1007/s00253-012-4502-5.
Katsuda, T., Lababpour, A., Shimahara, K., & Katoh, S. (2004). Astaxanthin production by Haematococcus pluvialis under illumination with LEDs. Enzyme and Microbial Technology, 35(1), 81–86. https://doi.org/10.1016/j.enzmictec.2004.03.016.
Baba, M., Kikuta, F., Suzuki, I., Watanabe, M. M., & Shiraiwa, Y. (2012). Wavelength specificity of growth, photosynthesis, and hydrocarbon production in the oil-producing green alga Botryococcus braunii. Bioresource Technology, 109, 266–270. https://doi.org/10.1016/j.biortech.2011.05.059.
Acknowledgments
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2017/24486-0) for the fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cezare-Gomes, E.A., Mejia-da-Silva, L.d., Pérez-Mora, L.S. et al. Potential of Microalgae Carotenoids for Industrial Application. Appl Biochem Biotechnol 188, 602–634 (2019). https://doi.org/10.1007/s12010-018-02945-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-02945-4