Abstract
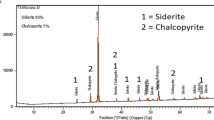
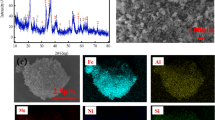
The leaching kinetics of low-grade copper ore with high-alkality gangues was studied in ammonia-ammonium sulphate solution. The main parameters, such as ammonia and ammonium sulphate concentrations, particle size, solid-to-liquid ratio and reaction temperature, were chosen in the experiments. The results show that the increase of temperature, concentrations of ammonia and ammonium sulphate is propitious to the leaching rate of copper ore. The leaching rate increases with the decrease of particle size and solid-to-liquid ratio. The leaching rate is controlled by the diffusion through the ash layer and the activation energy is determined to be 25.54 kJ/mol. A semi-empirical equation was proposed to describe the leaching kinetics.
Similar content being viewed by others
References
CAO Zhan-fang, ZHONG Hong, LIU Guang-yi, ZHAO Shu-juan. Techniques of copper from Mexican copper oxide ore [J]. Mining Science and Technology, 2009, 19(1): 45–48.
ZHANG Jie, WU Ai-xiang, WANG Yi-ming, CHEN Xue-song. Experimental research in leaching of copper-bearing tailings enhanced by ultrasonic treatment [J]. J China Univ Ming and Technol, 2008, 18(1): 98–102.
WATLING H R. The bioleaching of sulphide minerals with emphasis on copper sulphites-A review [J]. Hydrometallurgy, 2006, 84: 81–108.
ANTONIJEVIĆ M M, DIMITRIJEVIĆ M D, STEVANOVIĆ Z O. Investigation of possibility of copper recovery from the floatation tailing by acid leaching [J]. Journal of Hazardous Materials, 2008, 158(1): 23–34.
PARK K H, MOHAPATRA D, REDDY B R, NAM C W. A study on the oxidative ammonia/ammonium sulphate leaching of a complex (Cu-Ni-Co-Fe) matte [J]. Hydrometallurgy, 2007, 86(3/4): 164–171.
LWAMBIYI M, MAWEJA K, KONGOLO K, LWAMBIYI N M, DIYAMBI M. Investigation into the leap leaching of copper ore from the Disele deposit [J]. Hydrometallurgy, 2008, 98(1/2): 177–180.
LIU Wei, TANG Mo-tang, TANG Chao-bo, HE Jing, YANG Sheng-hai, YANG Jian-guang. Thermodynamics of solubility of Cu2(OH)2CO3 in ammonia-ammonium chloride-ethylene-diamine (En)-water system [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(2): 336–343.
SOKIĆ M D, MARKOVIĆ B, ŽIVKOVIĆ D. Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid [J]. Hydrometallurgy, 2009, 95(3/4): 273–279.
SUN Xi-liang, CHEN Bai-zhen, YANG Xi-yun, LIU You-yuan. Technological conditions and kinetics of leaching copper from complex copper oxide ore [J]. Journal of Central South University of Technology, 2009, 16(6): 936–941.
BRYDEN K O. Ammonium sulphate leaching of malachite and chrysocolla [D]. Salt Lake City: Department of Metallurgical Engineering, University of Utah, 1975: 25–48.
OUDENNE P D, OLSON F A. Leaching kinetics of malachite in ammonium carbonate solution [J]. Metallurgical Transaction B, 1983, 14(1): 33–40.
EKMEKYAPAR A, OYA R. Dissolution kinetics of an oxidized copper ore in ammonium chloride solution [J]. Chemical and Biochemical Engineering Quarterly, 2003, 17(4): 261–266.
KÜNKÜL M M, KOCAKERIM S, DEMIRBAĜ Y A. leaching kinetics of malachite in ammonia solution [J]. International Journal of Mineral Processing, 1994, 41(3): 167–182.
BINGÖL D, CANBAZOĜL M, AYDIĜAN S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching [J]. Hydrometallurgy 2005, 76(1/2): 55–62.
JU Shao-hua. Study in hydrometally thermodynamics of metal (Cu, Ni, Au) in the system of Me-NH4Cl-NH3-H2O and heap leaching process of their low-grade ores [D]. Changsha: School of Metallurgical Science and Engineering, Central South University, 2006: 50–51. (in Chinese)
LEVENSPIEL O. Chemical reaction engineering [M]. New York: Wiley, 1972: 361–371.
JACKSON E. Hydrometallurgical extraction and reclamation [M]. Chichester: Eillis Horwood Ltd., 1982: 46–47.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2007CB613601) supported by the National Basic Research Program of China; Project(10C1095) supported by the Foundation of Hunan Educational Committee, China
Rights and permissions
About this article
Cite this article
Liu, Zx., Yin, Zl., Hu, Hp. et al. Leaching kinetics of low-grade copper ore with high-alkality gangues in ammonia-ammonium sulphate solution. J. Cent. South Univ. Technol. 19, 77–84 (2012). https://doi.org/10.1007/s11771-012-0975-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-012-0975-8