Abstract
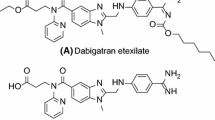
To obtain a highly selective, direct coagulation factor Xa (FXa) inhibitor with excellent antithrombotic activity and improved solubility, a series of novel 3,4-diaminobenzoyl derivatives were designed and synthesized based on reported structure–activity relationship analysis of FXa inhibitors. Preliminary solubility test showed that the decrease of intramolecular hydrogen bonds and the change of rigid structure could effectively improve physicochemical property of compounds. The synthesized compound 7ab could significantly prolong prothrombin time at a concentration of 40 nM compared with Apixaban. Docking investigation of 7ab with FXa protein revealed that the several groups could form multiple hydrogen bonds with amino acid residues ALA-190, ASP-189 and GLY-216. These results indicated that compound 7ab might serve as a potential anticoagulant agent.



Similar content being viewed by others
References
Al-Horani RA, Mehta AY, Desai UR (2012) Potent direct inhibitors of factor Xa based on the tetrahydroisoquinoline scaffold. Eur J Med Chem 54:771–783. https://doi.org/10.1016/j.ejmech.2012.06.032
Bijak M, Bobrowski M, Borowiecka M, Podsędek A, Golański J, Nowak P (2011) Anticoagulant effect of polyphenols-rich extracts from black chokeberry and grape seeds. Fitoterapia 82:811–817. https://doi.org/10.1016/j.fitote.2011.04.017
Bijak M, Ponczek MB, Nowak P (2014) Polyphenol compounds belonging to flavonoids inhibit activity of coagulation factor X. Int J Biol Macromol 65:129–135. https://doi.org/10.1016/j.ijbiomac.2014.01.023
Bijak M, Saluk J, Szelenberger R, Nowak P (2016) Popular naturally occurring antioxidants as potential anticoagulant drugs. Chem Biol Interact 257:35–45. https://doi.org/10.1016/j.cbi.2016.07.022
Davie EW, Ratnoff OD (1964) Waterfall sequence for intrinsic blood clotting. Science 145:1310–1312. https://doi.org/10.1126/science.145.3638.1310
de Candia M et al (2013) Synthesis and biological evaluation of direct thrombin inhibitors bearing 4-(piperidin-1-yl)pyridine at the P1 position with potent anticoagulant activity. J Med Chem 56:8696–8711. https://doi.org/10.1021/jm401169a
Evano G, Blanchard N, Toumi M (2008) Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chem Rev 108:3054–3131. https://doi.org/10.1021/cr8002505
Imaeda Y et al (2008) Discovery of imidazo[1,5-c]imidazol-3-ones: weakly basic, orally active factor Xa inhibitors. J Med Chem 51:3422–3436. https://doi.org/10.1021/jm701548u
Kleanthous S et al (2010) Structure and property based design of factor Xa inhibitors: pyrrolidin-2-ones with monoaryl P4 motifs. Bioorg Med Chem Lett 20:618–622. https://doi.org/10.1016/j.bmcl.2009.11.077
Kohrt JT et al (2006) The discovery of glycine and related amino acid-based factor Xa inhibitors. Bioorg Med Chem 14:4379–4392. https://doi.org/10.1016/j.bmc.2006.02.040
Kohrt JT et al (2007) The discovery of (2R,4R)-N-(4-chlorophenyl)-N-(2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl)-4-methoxypyrrolidine-1,2-dicarboxamide (PD 0348292), an orally efficacious factor Xa inhibitor. Chem Biol Drug Des 70:100–112. https://doi.org/10.1111/j.1747-0285.2007.00539.x
Lee YK et al (2008) 7-Fluoroindazoles as potent and selective inhibitors of factor Xa. J Med Chem 51:282–297. https://doi.org/10.1021/jm701217r
Macfarlane RG (1964) An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature 202:498. https://doi.org/10.1038/202498a0
Matter H et al (2002) Design and quantitative structure-activity relationship of 3-amidinobenzyl-1H-indole-2-carboxamides as potent, nonchiral, and selective inhibitors of blood coagulation factor Xa. J Med Chem 45:2749–2769
Nagahara T et al (1994) Dibasic (amidinoaryl)propanoic acid derivatives as novel blood coagulation factor Xa inhibitors. J Med Chem 37:1200–1207
Nazare M et al (2005) Probing the subpockets of factor Xa reveals two binding modes for inhibitors based on a 2-carboxyindole scaffold: a study combining structure–activity relationship and X-ray crystallography. J Med Chem 48:4511–4525. https://doi.org/10.1021/jm0490540
Pruitt JR et al (2000) Isoxazolines and isoxazoles as factor Xa inhibitors. Bioorg Med Chem Lett 10:685–689
Qiao JX et al (2007) SAR and X-ray structures of enantiopure 1,2-cis-(1R,2S)-cyclopentyldiamine and cyclohexyldiamine derivatives as inhibitors of coagulation Factor Xa. Bioorg Med Chem Lett 17:4419–4427. https://doi.org/10.1016/j.bmcl.2007.06.029
Trott O, Olson JA (2009) Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. https://doi.org/10.1002/jcc.21334
Van Huis CA et al (2009) Exploration of 4,4-disubstituted pyrrolidine-1,2-dicarboxamides as potent, orally active factor Xa inhibitors with extended duration of action. Bioorg Med Chem 17:2501–2511. https://doi.org/10.1016/j.bmc.2009.01.063
Yang J, Su G, Ren Y, Chen Y (2015) Synthesis of 3,4-diaminobenzoyl derivatives as factor Xa inhibitors. Eur J Med Chem 101:41–51. https://doi.org/10.1016/j.ejmech.2015.06.012
Young RJ et al (2006) Structure- and property-based design of factor Xa inhibitors: pyrrolidin-2-ones with acyclic alanyl amides as P4 motifs. Bioorg Med Chem Lett 16:5953–5957. https://doi.org/10.1016/j.bmcl.2006.09.001
Young RJ et al (2011) Structure and property based design of factor Xa inhibitors: pyrrolidin-2-ones with aminoindane and phenylpyrrolidine P4 motifs. Bioorg Med Chem Lett 21:1582–1587. https://doi.org/10.1016/j.bmcl.2011.01.131
Zhao Y et al (2015) Design, synthesis and structure–activity relationship of oxazolidinone derivatives containing novel S4 ligand as FXa inhibitors. Eur J Med Chem 96:369–380. https://doi.org/10.1016/j.ejmech.2015.04.025
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, G., Yang, J., Su, B. et al. Design and synthesis of novel 3,4-diaminobenzoyl derivatives as antithrombotic agents with improved solubility. Chem. Pap. 73, 987–994 (2019). https://doi.org/10.1007/s11696-018-0645-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0645-x