Abstract
Background
Retrospective studies have found that early tumor shrinkage (ETS) and depth of response (DpR) are associated with favorable outcomes in patients with metastatic colorectal cancer (mCRC); however, few prospective studies have evaluated ETS and DpR.
Patients and Methods
We performed a phase II study of FOLFOX plus cetuximab as first-line treatment in Japanese patients with KRAS wild-type mCRC. The primary endpoint was response rate (RR), and secondary endpoints included progression-free survival (PFS), overall survival (OS), chronological tumor shrinkage (evaluated every 8 weeks), and safety. The association of ETS and DpR with survival time was analyzed using Spearman’s rank correlation coefficient.
Results
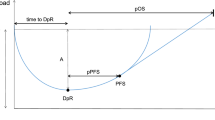
In 54 participants, the RR, median PFS, and OS were 66.7 % (95 % CI, 53.4–77.8 %), 11.1 months, and 33.9 months, respectively. There was no unexpected toxicity. Forty (80 %) of 50 assessable patients had ETS, which was associated with prolonged PFS and OS (11.3 vs. 3.7 months, HR 0.26, p = 0.0003; 42.8 vs. 9.0 months, HR 0.40, p = 0.0279, respectively). Median DpR was 56.3 %. The DpR correlated with OS (r s = 0.314, p = 0.027) as well as post-progression survival (PPS) (r s = 0.366, p = 0.017). Interestingly, DpR was moderately associated with OS and PPS (r s = 0.587, r s = 0.570, respectively) in patients harboring tumors with larger target lesions, but was not associated with OS or PPS in patients with smaller target lesions. FOLFOX plus cetuximab was active as a first-line treatment for Japanese mCRC patients, with no unexpected toxicities.
Conclusions
Our prospective evaluation of chronological tumor shrinkage showed that ETS and DpR correlate with outcomes in patients with KRAS wild-type mCRC who receive cetuximab-based chemotherapy (UMIN000004197).


Similar content being viewed by others
References
Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer. 2001;92:1331–46.
Saltz LB, Meropol NJ, Loehrer Sr PJ, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8.
Folprecht G, Lutz MP, Schoffski P, Seufferlein T, Nolting A, Pollert P, et al. Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol. 2006;17:450–6.
Galizia G, Lieto E, De Vita F, Orditura M, Castellano P, Troiani T, et al. Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. 2007;26:3654–60.
Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–8.
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17.
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71.
Heinemann V, Stintzing S, Modest DP, Giessen-Jung C, Michl M, Mansmann UR. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer. 2015;51:1927–36.
Giessen C, Laubender RP, von Fischervon WL, Schalhorn A, Modest DP, Stintzing S, et al. Early tumor shrinkage in metastatic colorectal cancer: retrospective analysis from an irinotecan-based randomized first-line trial. Cancer Sci. 2013;104:718–24.
Modest DP, Laubender RP, Stintzing S, Giessen C, Schulz C, Haas M, et al. Early tumor shrinkage in patients with metastatic colorectal cancer receiving first-line treatment with cetuximab combined with either CAPIRI or CAPOX: an analysis of the German AIO KRK 0104 trial. Acta Oncol. 2013;52:956–62.
Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26:1188–94.
Piessevaux H, Buyse M, Schlichting M, Van Cutsem E, Bokemeyer C, Heeger S, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013;31:3764–75.
Mansmann UR, Sartorius U, Laubender RP, Giessen CA, Esser R, Heinemann V. Quantitative analysis of the impact of deepness of response on post-progression survival time following first-line treatment in patients with mCRC. Ann Oncol. 2013;24 Suppl 4:iv14-iv15.
Fakih M, Wong R. Efficacy of the monoclonal antibody EGFR inhibitors for the treatment of metastatic colorectal cancer. Curr Oncol. 2010;17 Suppl 1:S3–17.
Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535–46.
Lenz H-J, Lee F-C, Yau L, Koh HA, Knost JA, Mitchell EP, et al. MAVERICC, a phase 2 study of mFOLFOX6-bevacizumab (BV) vs FOLFIRI-BV with biomarker stratification as first-line (1L) chemotherapy (CT) in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol. 2016;34 Suppl 4S:abstr 493.
Stintzing S, Modest DP, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S, Heintges T, Lerchenmueller C, Kahl C, Seipelt G, Kullmann F, Scheithauer W, Held S, Giessen C, Moehler M, Jagenburg A, Jung A, Kirchner T, Heinemann V. Independent radiological evaluation of objective response, early tumor shrinkage, and depth of response in FIRE-3 (AIO KRK-0306) in the final RAS evaluable population. Ann Oncol. 2014;25 Suppl 5:v1-v41.
Ichante J, Adenis A, Malka D, Francois E, Boucher E, Chauffert B, Pignon J, Ychou M, Pierga J, Montoto-Grillot C, Conroy T, Ducreux M, GI group of FNCLCC. Impact of early tumor shrinkage on long-term outcome in metastatic colorectal cancer (mCRC) treated with 5FU plus irinotecan plus leucovorin (FOLFIRI) or capecitabine plus irinotecan XELIRI plus bevacizumab. J Clin Oncol. 2011;29 Suppl:abstr e 14041.
Sommeijer DW, Shi Q, Meyers JP, Sjoquist KM, Hoff PM, Seymour MT, Cassidy J, Goldberg RM, Douillard J-Y, Hecht JR, Hurwitz H, Tournigand C, Tebbutt NC, Aranda E, Souglakos J, Kabbinavar FF, Chibaudel B, De Gramont A, Sargent DJ, Zalcberg JR, for the ARCAD Group. Prognostic value of early objective tumor response (EOTR) to first-line systemic therapy in metastatic colorectal cancer (mCRC): Individual patient data (IPD) meta-analysis of randomized trials from the ARCAD database. J Clin Oncol. 2013;31 Suppl:abstr 3520.
Acknowledgments
We thank the patients, their families, and the investigators who participated in the JACCRO CC-05 trial. We also thank Sachika Koyama for editorial assistance and Peter Star (Medical Network K.K.) and Martin D. Berger for English editorial support. Akihito Tsuji and Yu Sunakawa contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflict of Interest
Author A.T. has received honoraria from Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma, and Merck Serono. Author Y.S. has received honoraria from Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma, Yakult Honsha, and Merck Serono. Author W.I. has received consulting fees from Merck Serono, Daiichi Sankyo, Zeria Pharmaceutical, and Ono Pharmaceutical; research funding from Merck Serono, Taiho Pharmaceutical, Takeda, and Eisai; and honoraria from Taiho Pharmaceutical, Merck Serono, Chugai Pharma, Daiichi Sankyo, Takeda, Nippon Kayaku, and Sawai Pharmaceutical Co. Author M.N. has received honoraria from Merck Serono, Takeda, Chugai Pharma, Taiho Pharmaceutical, Nihonkayaku, Novartis, Yakult Honsha, Lilly Japan, Bristol-Myers Squibb, Bayer, Ajinomoto, Shionogi, Pfizer, and Ono Pharmaceutical. Author M.K. has received honoraria from Merck Serono, Takeda, Chugai Pharma, and Yakult Honsha. Author M.T. has received consulting fees from Taiho Pharmaceutical, Shionogi, AbbVie GK, AstraZeneca, and Hisamitsu Pharma Co. ; honoraria from Mitsubishi Tanabe Pharma. Author M.F. has received consulting fees from Taiho Pharmaceutical. The other authors have declared no conflicts of interest.
Funding
This study was funded by the Japan Clinical Cancer Research Organization (JACCRO).
Rights and permissions
About this article
Cite this article
Tsuji, A., Sunakawa, Y., Ichikawa, W. et al. Early Tumor Shrinkage and Depth of Response as Predictors of Favorable Treatment Outcomes in Patients with Metastatic Colorectal Cancer Treated with FOLFOX Plus Cetuximab (JACCRO CC-05). Targ Oncol 11, 799–806 (2016). https://doi.org/10.1007/s11523-016-0445-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-016-0445-6