Abstract
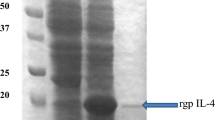
Interleukin-2 (IL-2) is a vital cytokine secreted by activated T lymphocytes, and plays an important role in the regulation of cellular and immunity of animals. In this study, a gene encoding duck IL-2 was cloned and the soluble recombinant duck IL-2 (rDuIL-2) was expressed in Escherichia coli via fusion with glutathione S-transferase (GST). The results indicated that the GST-rDuIL-2 fusion protein expressed in E. coli Origami (DE3) was confirmed to be of about 40 kDa molecular mass by SDS-PAGE and western blotting. In order to produce soluble rDuIL-2 in a low-cost, nontoxic and high-level expression process, lactose was used as a substitute for Isopropyl-β-D-thiogalactopyranoside (IPTG) to induce the above recombinant strain Origami (pGEX-DuIL-2). By optimizing the expression conditions, the production of soluble GST-rDuIL-2 fusion protein was about 29% of total cellular soluble proteins, which was similar with IPTG used as inducer. The soluble GST-rDuIL-2 fusion protein was purified by one-step affinity chromatography, and GST was removed by thrombin. Then rDuIL-2 was purified by a second affinity chromatography. As a result, the 95% pure rduIL-2 was obtained, and the yield of rDuIL-2 was about 10.6 mg/l bacterial culture. The bioactivity of rduIL-2 was determined by lymphocyte proliferation assay in vitro. Our study provided a feasible and convenient approach to produce soluble and biologically active rDuIL-2, which would be used as an immunoadjuvants for enhancing vaccine efficacy.






Similar content being viewed by others
References
Abubakar MB, Aini I, Omar AR, Hair-Bejo M (2011) Cloning and expression of highly pathogenic Avian Influenza virus full-length nonstructural gene in Pichia pastoris. J Biomed Biotechnol 2011:1–5
Antony PA, Paulos CM, Ahmadzadeh M, Akpinarli A, Palmer DC, Sato N, Kaiser A, Hinrichs CS, Klebanoff CA, Tagaya Y, Restifo NP (2006) Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J Immunol 176:5255–5266
Bessette PH, Aslund F, Beckwith J, Georgiou G (1999) Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc Natl Acad Sci USA 96:13703–13708
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao MJ, Jimmy K (2004) Monoclonal antibody production and purification of native chicken interleukin-2. Pharm Biotechnol 11:232–237
Du CH, Yi XP, Zhang YX (2010) Expression and purification of soluble recombinant human endostatin in Escherichia coli. Biotechnol Bioprocess Eng 15:229–235
Fei YF, Qian JF, Ling W, Li Y, Zhang ZB, Wang GS, Li XR (2004) Immune enhancing effect of recombinant IL-2 on a four in one oil adjuvant inactivited vaccine. Animal Husb Vet Med 36:9–11
Guo C, Zheng ZH, Wu GP, Lin Y, Su WJ, Cao MJ (2006) Study on the cloning, expression and function of chicken interleukin-2. J Fuzhou Univ (Nat Sci Ed) 34:760–765
Liang CM, Tai MC, Chang YH, Chen YH, Chen CL, Chien MW, Chen JT (2010) Glucosamine inhibits epidermal growth factor-induced proliferation and cell-cycle progression in retinal pigment epithelial cells. Mol Vis 16:2559–2571
Schmohl K, Schultz U (2000) Cloning and characterization of duck interleukin-2. GenBank accession no. AF294323
Sreekumar E, Premraj A, Rasool TJ (2005) Duck (Anas platyrhynchos), Japanese quail (Coturnix coturnix japonica) and other avian interleukin-2 reveals significant conservation of gene organization, promoter elements and functional residues. Eur J Immunogenet 32:355–365
Wang JY, Qi J, Fang J, Yan Y, Shen HG, Zhou JY (2008) Full-length genome organization analysis of duck interleukin-2. Chin J Vet Sci 28:15–19
Zhou JY, Chen JG, Wang JY, Kwang J (2003) Cloning and genetic evolution analysis of chIL-2 gene of Chinese local breeds. Prog Biochem Biophys 30:384–389
Zhou JY, Cheng LQ, Zheng XJ, Wu JX, Shang SB, Wang JY, Chen JG (2004) Generation of the transgenic potato expressing fulllength spike protein of infectious bronchitis virus. J Biotechnol 111:121–130
Zhou JY, Chen JG, Wang JY, Wu JX, Gong H (2005a) cDNA cloning and functional analysis of goose interleukin-2. Cytokine 30:328–338
Zhou JY, Wang JY, Chen JG, Wu JX, Gong H, Teng QY, Guo JQ, Shen HG (2005b) Cloning, in vitro expression and bioactivity of duck interleukin-2. Mol Immunol 42:589–598
Acknowledgments
This study was supported by the Key Research Foundation of Fujian provincial department of Science and Technology, China (2009N0042) and the Fujian Provincial Nature Science Foundation (2010J01212).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
du, C., Han, L. & Xie, Z. Cloning, expression in Escherichia coli, and purification of soluble recombinant duck interleukin-2. World J Microbiol Biotechnol 28, 1495–1501 (2012). https://doi.org/10.1007/s11274-011-0951-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0951-8