Abstract
Vitamin E comprises a group of eight lipid soluble antioxidant compounds that are an essential part of the human diet. The α-isomers of both tocopherol and tocotrienol are generally considered to have the highest antioxidant activities. γ-tocopherol methyltransferase (γ-TMT) catalyzes the final step in vitamin E biosynthesis, the methylation of γ- and δ-isomers to α- and β-isomers. In present study, the Arabidopsis γ-TMT (AtTMT) cDNA was overexpressed constitutively or in the endosperm of the elite japonica rice cultivar Wuyujing 3 (WY3) by Agrobacterium-mediated transformation. HPLC analysis showed that, in brown rice of the wild type or transgenic controls with empty vector, the α-/γ-tocotrienol ratio was only 0.7, much lower than that for tocopherol (~19.0). In transgenic rice overexpressing AtTMT driven by the constitutive Ubi promoter, most of the γ-isomers were converted to α-isomers, especially the γ- and δ-tocotrienol levels were dramatically decreased. As a result, the α-tocotrienol content was greatly increased in the transgenic seeds. Similarly, over-expression of AtTMT in the endosperm also resulted in an increase in the α-tocotrienol content. The results showed that the α-/γ-tocopherol ratio also increased in the transgenic seeds, but there was no significant effect on α-tocopherol level, which may reflect the fact that γ-tocopherol is present in very small amounts in wild type rice seeds. AtTMT overexpression had no effect on the absolute total content of either tocopherols or tocotrienols. Taken together, these results are the first demonstration that the overexpression of a foreign γ-TMT significantly shift the tocotrienol synthesis in rice, which is one of the world’s most important food crops.




Similar content being viewed by others
References
Aitzetmüller K (1997) Antioxidative effects of carum seeds. J Am Oil Chem Soc 74:185
Bramley PM, Elmadfa I, Kafatos A, Kelly FJ, Manios Y, Roxborough HE, Schuch W, Sheehy PJA, Wagner KH (2000) Vitamin E. J Sci Food Agric 80:913–938
Britz SJ, Prasad PVV, Moreau RA, Allen LHJ, Kremer DF, Boote KJ (2007) Influence of growth temperature on the amounts of tocopherols, tocotrienols, and γ-oryzanol in brown rice. J Agric Food Chem 55:7559–7565
Buring JE, Hennekens CH (1997) Antioxidant vitamins and cardiovascular disease. Nutr Rev 55:S53–S60
Cahoon EB, Hall SE, Ripp KG, Ganzke TS, Hitz WD, Coughlan SJ (2003) Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat Biotech 21:1082–1087
Chaudhary N, Khurana P (2009) Vitamin E biosynthesis genes in rice: molecular characterization, expression profiling and comparative phylogenetic analysis. Plant Sci 177:479–491
Collakova E, DellaPenna D (2003) Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol 131:632–642
Crowell EF, McGrath JM, Douches DS (2008) Accumulation of vitamin E in potato (Solanum tuberosum) tubers. Transgenic Res 17:205–217
DellaPenna D (2005) Progress in the dissection and manipulation of vitamin E synthesis. Trends Plant Sci 10:574–579
Falk J, Munné-Bosch S (2010) Tocochromanol functions in plants: antioxidation and beyond. J Exp Bot 61:1549–1566
Falk J, Brosch M, Schäfer A, Braun S, Krupinska K (2005) Characterization of transplastomic tobacco plants with a plastid localized barley 4-hydroxy- phenylpyruvate dioxygenase. J Plant Physiol 162:738–742
Farré G, Sudhakar D, Naqvi S, Sandmann G, Christou P, Capell T, Zhu C (2012) Transgenic rice grains expressing a heterologous ρ-hydroxyphenylpyruvate dioxygenase shift tocopherol synthesis from the γ to the α isoform without increasing absolute tocopherol levels. Transgenic Res. doi:10.1007/s11248-012-9601-7
Heinemann RJB, Xu Z, Godber JS, Lanfer-Marquez UM (2008) Tocopherols, tocotrienols, and γ-oryzanol contents in japonica and indica subspecies of rice (Oryza sativa L.) cultivated in Brazil. Cereal Chem 85:243–247
Hofius D, Hajirezaei MR, Geiger M, Tschiersch H, Melzer M, Sonnewald U (2004) RNAi-mediated tocopherol deficiency impairs photo assimilate export in transgenic potato plants. Plant Physiol 135:1256–1268
Huang SH, Ng LT (2011) Quantification of tocopherols, tocotrienols, and γ-oryzanol contents and their distribution in some commercial rice varieties in Taiwan. J Agric Food Chem 59:11150–11159
Hunter SC, Cahoon EB (2007) Enhancing vitamin E in oilseeds: unraveling tocopherol and tocotrienol biosynthesis. Lipids 42:97–108
Ikeda I, Imasato Y, Sasaki E, Sugano M (1996) Lymphatic transport of alpha-, gamma- and delta-tocotrienols and alpha-tocopherol in rats. Int J Vitam Nutr Res 66:217–221
Kamal-Eldin A, Appelqvist LA (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31:671–701
Kannappan R, Gupta SC, Kim JH, Aggarwal BB (2012) Tocotrienols fight cancer by targeting multiple cell signaling pathways. Genes Nutr 7:43–52
Karunanandaa B, Qi Q, Hao M, Baszis SR, Jensen PK, Wong YH, Jiang J, Venkatramesh M, Gruys KJ, Moshiri F, Post-Beittenmiller D, Weiss JD, Valentin HE (2005) Metabolically engineered oilseed crops with enhanced seed tocopherol. Metab Eng 7:384–400
Khanna S, Roy S, Slivka A, Craft TK, Chaki S, Rink C, Notestine MA, DeVries AC, Parinandi NL, Sen CK (2005) Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke 36:2258–2264
Khanna S, Roy S, Parinandi NL, Maurer M, Sen CK (2006) Characterization of the potent neuroprotective properties of the natural vitamin E alpha-tocotrienol. J Neurochem 98:1474–1486
Lee BK, Kim SL, Kim KH, Yu SH, Lee SC, Zhang Z, Kim MS, Park HM, Lee JY (2008) Seed specific expression of perilla γ-tocopherol methyltransferase gene increases α-tocopherol content in transgenic perilla (Perilla frutescens). Plant Cell Tiss Organ Cult 92:47–54
Li Y, Wang Z, Sun X, Tang K (2008) Current opinions on the functions of tocopherol based on the genetic manipulation of tocopherol biosynthesis in plants. J Integr Plant Biol 50:1057–1069
Li Y, Wang G, Hou R, Zhou Y, Gong R, Sun X, Tang K (2011) Engineering tocopherol biosynthetic pathway in lettuce. Biol Plant 55:453–460
Liu QQ, Zhang JL, Wang ZY, Hong MM, Gu MH (1998) A highly efficient transformation mediated by Agrobacterium in rice. Acta Phytophysiol Sin 24:259–271
Maeda H, DellaPenna D (2007) Tocopherol functions in photosynthetic organisms. Curr Opin Plant Biol 10:260–265
Miyazawa T, Shibata A, Sookwong P, Kawakami Y, Eitsuka T, Asai A, Oikawa S, Nakagawa K (2009) Antiangiogenic and anticancer potential of unsaturated vitamin E (tocotrienol). J Nutr Biochem 20:79–86
Munné-Bosch S, Peñuelas J (2003) Photo- and antioxidative protection during summer leaf senescence in Pistacia lentiscus L. grown under Mediterranean field conditions. Ann Bot 92:385–391
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Naqvi S, Farré G, Zhu CF, Sandmann G, Capell T, Christou P (2011) Simultaneous expression of Arabidopsis ρ-hydroxyphenylpyruvate dioxygenase and MPBQ methyltransferase in transgenic corn kernels triples the tocopherol content. Transgenic Res 20:177–181
Osakada F, Hashino A, Kume T, Katsuki H, Kaneko S, Akaike A (2004) Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology 47:904–915
Padley FB, Gunstone FD, Harwood JL (1994) Occurrence and characteristics of oils and fats. In: Gunstone FD, Harwood JL, Padley FB (eds) The lipid handbook, 2nd edn. Chapman & Hall, London, pp 127–130
Panfili G, Fratianni A, Irano M (2003) Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in Cereals. J Agric Food Chem 51:3940–3944
Park HA, Kubicki N, Gnyawali S, Chan YC, Roy S, Khanna S, Sen CK (2011) Natural vitamin E α-tocotrienol protects against ischemic stroke by induction of multidrug resistance-associated protein 1. Stroke 42:2308–2314
Rink C, Christoforidis G, Khanna S, Peterson L, Patel Y, Khanna S, Abduljalil A, Irfanoglu O, Machiraju R, Bergdall VK, Sen CK (2011) Tocotrienol vitamin E protects against preclinical canine ischemic stroke by inducing arteriogenesis. J Cereb Blood Flow Metab 31:2218–2230
Russell DA, Fromm ME (1997) Tissue-specific expression in transgenic maize of four endosperm promoters from maize and rice. Transgenic Res 6:157–168
Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16:1419–1432
Sen CK, Khanna S, Roy S (2006) Tocotrienols: vitamin E beyond tocopherols. Life Sci 78:2088–2098
Serbinova E, Kagan V, Han D, Packer L (1991) Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med 10:263–275
Sheppard AJ, Pennington JAT, Weihrauch JL (1993) Analysis and distribution of vitamin E in vegetable oils and foods. In: Packer L, Fuchs J (eds) Vitamin E in health and disease. Marcel Dekker, New York, pp 9–31
Shin TS, Godber JS, Martin DE, Wells JH (1997) Hydrolytic stability and changes in E vitamers and oryzanol of extruded rice bran during storage. J Food Sci 62:704–728
Shintani D, DellaPenna D (1998) Elevating the vitamin E content of plants though metabolic engineering. Science 282:2098–2100
Suzuki YJ, Tsuchiya M, Wassall SR, Choo YM, Govil G, Kagan VE, Packer L (1993) Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: implication to the molecular mechanism of their antioxidant potency. Biochemistry 32:10692–10699
Tangney CC (1997) Vitamin E and cardiovascular disease. Nutr Today 32:13–22
Tavva VS, Kim YH, Kagan IA, Dinkins RD, Kim KH, Collins GB (2007) Increased alpha-tocopherol content in soybean seed overexpressing the Perilla frutescens gamma-tocopherol methyltransferase gene. Plant Cell Rep 26:61–70
Van Eenennaam AL, Lincoln K, Durrett TP, Valentin HE, Shewmaker CK, Thorne GM, Jiang J, Baszis SR, Levering CK, Aasen ED, Hao M, Stein JC, Norris SR, Last RL (2003) Engineering vitamin E content: from Arabidopsis mutant to soy oil. Plant Cell 15:3007–3019
Yuen KH, Wong JW, Lim AB, Ng BH, Choy WP (2011) Effect of mixed-tocotrienols in hypercholesterolemic subjects. Funct Foods Health Dis 3:106–117
Yusuf MA, Sarin NB (2007) Antioxidant value addition in human diets: genetic transformation of Brassica juncea with γ-TMT gene for increased α-tocopherol content. Transgenic Res 16:109–113
Zhang GY, Liu RR, Zhang P, Xu Y, Zhu J, Gu MH, Liang GH, Liu QQ (2012) Variation and distribution of vitamin E and composition in the seeds among different rice varieties. Acta Agrono Sin 38:55–61
Zheng FQ, Wang ZY, Gao JP (1993) Isolation of total RNA from rice endosperm. Plant Physiol Commun 29:438–440
Acknowledgments
This study was supported by the National Key Basic Research Projects (2012CB944803), the National Special Program for Transgenic Research (2011ZX08001-006), and the Funds for Distinguished Young Scientists and Priority Academic Program Development from Jiangsu Government, China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11248_2012_9630_MOESM1_ESM.tif
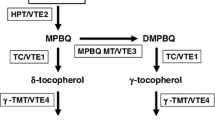
Supplemental Figure 1. Vitamin E biosynthetic pathway in plants. HPPD, p-hydroxyphenyl-pyruvate dioxygenase; HPT, homogentisate phytyltransferase; TC, tocopherol cyclase; TMT, γ-tocopherol methyltransferase; HGGT, homogentisate geranylgeranyl transferase; MPBQ MT2, methylphytylbenzoquinone methyltransferase. (TIFF 240 kb)
11248_2012_9630_MOESM2_ESM.docx
Supplemental Table 1. Tocochromanol composition and content in AtTMT transgenic brown rice. Each value is the average ± SD of three independent experiments. WT, wild-type WY3; Lines C1 and C2 are transgenic control lines derived from empty vectors pUbi and pGt1, respectively; Lines 1-7 are pUbi-TMT-derived transgenic lines, and lines 8-13 are pGt1-TMT-derived transgenic lines. (DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Zhang, GY., Liu, RR., Xu, G. et al. Increased α-tocotrienol content in seeds of transgenic rice overexpressing Arabidopsis γ-tocopherol methyltransferase. Transgenic Res 22, 89–99 (2013). https://doi.org/10.1007/s11248-012-9630-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-012-9630-2