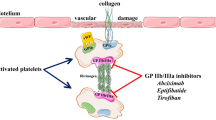
Abstract
The present review aims to describe the pharmacological aspects as well as the available clinical data supporting the choice of intracoronary route of administration for abciximab, an antiplatelet drug used in patients with acute coronary syndromes undergoing percutaneous coronary interventions (PCI). Abciximab is a glycoprotein (GP) IIb/IIIa receptor antagonist which determines a potent inhibition of platelet aggregation and thrombus formation. These properties seem to prevent not only thrombus formation but also to promote (at higher drug concentration) lysis of fresh thrombus. Moreover, differently from the other GP IIb/IIIa inhibitors, abciximab also binds to the vitronectin receptor on endothelial, smooth muscle, and inflammatory cells and to an activated conformation of the aMb2 receptor on leukocytes. Such cross-reactivity raises the possibility that clinical benefits derived from its use may not be exclusively due to its anti-thrombotic effect, but may also be related to the suppression of inflammatory pathways involving platelets, white blood cells, and the vascular endothelium. On such basis, the local administration of abciximab at the site of coronary thrombosis may enhance, by increasing its local concentration, the binding to both platelet and endothelium receptors. The results of several angiographic studies assessing the effect of intracoronary abciximab administration support on clinical grounds its adoption in patients with fresh coronary thrombosis. Indeed, better post-angioplasty coronary flow, greater degree of myocardial salvage and a better left ventricular function recovery have been achieved as compared to the intravenous, systemic, administration of drug’s bolus.
Condensed Abstract Several studies have highlighted the benefits of abciximab, a potent antiplatelet agent, in patients with acute coronary syndromes undergoing percutaneous coronary interventions. Moreover, differently from the other glycoprotein IIb/IIIa receptor antagonists, abciximab also has non-IIb/IIIa-related properties raising the possibility that clinical benefits derived from its use may not be exclusively due to its anti-thrombotic effect, but may also be related to the suppression of inflammatory pathways. Several angiographic studies in patients with fresh coronary thrombosis and recent clinical studies in patients with acute coronary syndromes undergoing mechanical revascularization support the hypothesis that local administration of abciximab at the site of the culprit coronary artery may facilitate both the de-thrombotic and the non-GP IIb/IIIa-dependent properties of the drug. On such basis, the present review aims to describe the pharmacological aspects as well as the available clinical data supporting the choice of intracoronary route of administration for abciximab.
Similar content being viewed by others
References
Larson RS, Springer TA (1990) Structure and function of leukocyte integrins. Immunol Rev 114:181–217
Reverter JC, Be´guin S, Kessels H, Kumar R, Hemker HC, Coller BS (1996) Inhibition of platelet-mediated, tissue factor-induced thrombin generation by the mouse/human chimeric 7E3 antibody. J Clin Invest 98:863–874
Topol EJ, Byzova TV, Plow EF (1999) Platelet GP IIb/IIIa blockers. Lancet 353:227–31
Theroux P, Kouz S, Roy L et al. (1996) Platelet membrane receptor glycoprotein IIb/IIIa antagonism in unstable angina: the Canadian Lamifiban study. Circulation 94:899–905
Schulman SP, Golschmidt-Clermont PJ, Topol EJ et al. (1996) Effects of integrelin, a platelet glycoprotein IIb/IIIa receptor antagonist, in unstable angina: a randomized multicenter trial. Circulation 94:2083–2089
The CAPTURE Investigators (1997) Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE study. Lancet 349:1429–1435
The EPIC Investigators (1994) Use of monoclonal antibody directed against the platelet glycoprotein receptor in high-risk coronary angioplasty. N Engl J Med 330:956–961
The EPILOG Investigators (1997) Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. N Engl J Med 336:1689–1696
Karvouni E, Katritsis DG, Ioannidis JP (2003) Intravenous glycoprotein IIb/IIIa receptor antagonists reduce mortality after percutaneous coronary interventions. J Am Coll Cardiol 41:26–32
Tcheng JE, Ellis SG, George BS et al (1994) Pharmacodynamics of chimeric glycoprotein IIb/IIIa integrin anti-platelet antibody Fab 7E3 in high risk coronary angioplasty. Circulation 90(4):1757–1764
Mascelli MA, Lance ET, Damaraju L, Wagner CL, Weisman HF, Jordan RE (1998) Pharmacodynamic profile of short-term abciximab treatment demonstrates prolonged platelet inhibition with gradual recovery from GPIIb/IIIa receptor blockade. Circulation 97:1680–1688
Simoons M, Jan de Boer M, Van den Brand M et al, and the European Cooperative Study Group (1994) Randomized trial of a GPIIB/IIIA platelet receptor blocker in refractory unstable angina. Circulation 89:596–603
Abernethy DR, Pezzullo J, Mascelli MA, Frederick B, Kleiman NS, Freedman J. (2002) Pharmacodynamics of abciximab during angioplasty: comparison to healthy subjects. Clin Pharmacol Ther 71(3):186–195
Wencel-Drake J, Plow E, Zimmerman T, Painter R, Ginsberg M (1984) Immunofluorescent localization of adhesive glycoprotein in resting and thrombinstimulated platelet. Am J Pathol 115:156–164
Niya K, Hodson E, Bader R et al (1987) Increased surface expression of the membrane glycoprotein IIb/IIIa complex induced by platelet activation, relationship to the binding of fibrinogen and platelet aggregation. Blood 70:475–483
Marciniak SJ Jr, Mascelli MA, Furman MI et al (2002) An additional mechanism of action of abciximab: dispersal of newly formed platelet aggregates. Thromb Haemost 87:1020–1025
Cox AD, Devine DV (1994) Factor XIIIa binding to activated platelets is mediated through activation of glycoprotein IIb-IIIa. Blood 83:1006–1016
Cohen I, Berk DL, White JG (1989) The effect of peptides and monoclonal antibodies that bind to platelet glycoprotein IIb/IIIa complex on the development of clot tension. Blood 73:1880–1887
Hantgan RR, Moussa SA (1998) Inhibition of platelet-mediated clot retraction by integrin antagonists. Thromb Res 89:271–279
Reverter JC, Beguin S, Kessels H, Kumar R, Hemker HC, Coller BS (1996) Inhibition of platelet-mediated, tissue factor-induced thrombin generation by the mouse/human chimeric 7E3 antibody. Potential implications for the effect of c7E3 Fab treatment on acute thrombosis and “clinical restenosis”. J Clin Invest 98(3):863–874
Collet JP, Mishal Z, Soria J, Soria C, Thomas D, Montalescot G (2001) Disaggregation of in vitro platelet-rich clots by abciximab increases fibrinogen exposure and promotes fibrinolysis. J Arth Vasc Biol 21:142–148
Hynes RO (1992) Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11–25
Hynes RO (1987) Integrins: a family of cell surface receptors. Cell 48:549–554
Tam SH, Sassoli PM, Jordan RE, Nakada MT (1998) Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent affinity and functional blockade of glycoprotein IIb/IIIa and αvβ3 integrins. Circulation 98:1085–1091
Altieri DC, Edgington TS (1988) A monoclonal antibody reacting with distinct adhesion molecules defines a transition in the functional state of the receptor CD11b/CD18 (Mac-1). J Immunol 141:2656–2660
Neumann FJ, Zohlnhofer D, Fakhoury L, Ott I, Gawaz M, Schomig A (1999) Effect of glycoprotein IIb/IIIa receptor blockade on platelet–leukocyte interaction and surface expression of the leukocyte integrin Mac-1 in acute myocardial infarction. J Am Coll Cardiol 34:1420–6
Duff GL, McMillan GC, Ritchie AC (1957) The morphology of early atherosclerotic lesions of the aorta demonstrated by the surface technique in rabbits fed cholesterol. Am J Pathol 33:845–873
Saphir O, Gore I (1950) Evidence for an inflammatory basis of coronary arteriosclerosis in the young. Arch Pathol 49:418–426
Martinet Y, Bitterman PB, Mornex J-F, Grotendorst GR, Martin GR, Crystal RG (1986) Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature 319:158–160
Rogers C, Welt FGP, Karnovsky MJ, Edelman ER (1996) Monocyte recruitment and neointimal hyperplasia in rabbits: coupled inhibitory effects of heparin. Arterioscler Thromb Vasc Biol 16:1312–1318
Coller BS (1999) Binding of abciximab to aVb3 and activated aMb2 receptors: with a review of platelet-leukocyte interactions. Thromb Haemost 82:326–335
Diamond MS, Staunton DE, Morlin SD, Springer TA (1991) Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell 65:961–971
Altieri DC, Bader R, Mannucci PM, Edgington TS (1988) Oligospecificity of the cellular adhesion receptor Mac-1 encompasses an inducible recognition specificity for fibrinogen. J Cell Biol 107:1893–1900
Altieri DC, Morrissey JH, Edgington TS (1988) Adhesive receptor Mac-1 coordinates the activation of factor X on stimulated cells of monocytic and myeloid differentiation: an alternative initiation of the coagulation cascade. Proc Natl Acad Sci USA 85:7462–7466
Rosen H, Gordon S (1987) Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J Exp Med 166:1685–1701
Vedder NB, Winn RK, Rice CL, Chi EY, Arfors KE, Harlan JM (1988) A monoclonal antibody to the adherence-promoting leukocyte glycoprotein, CD18, reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J Clin Invest 81:939–944
Simpson PJ, Todd RF, Fantone JC, Mickelson JK, Griffin JD, Lucchesi BR (1988) Reduction of experimental myocardial reperfusion injury by a monoclonal antibody (anti Mo1, anti CD11b) that inhibits leukocyte adhesion. J Clin Invest 81:624–629
Kassirer M, Zeltser D, Prochorov V et al (1999) Increased expression of the CD 11b/CD18 antigen on the surface of peripheral white blood cells in patients with ischemic heart disease: further evidence for smoldering inflammation in patients with atherosclerosis. Am Heart J 138:555–559
Mickelson JK, Ali MN, Kleiman NS et al (1999) Chimeric 7E3 Fab (ReoPro) decreases detectable CDllb on neutrophils from patients undergoing coronary angioplasty. J Am Coll Cardiol 33:97–106
Farb A, Sangiorgi G, Carter AJ et al (1999) Pathology of acute and chronic coronary stenting in humans. Circulation 99:44–52
Grewe PH, Deneke T, Machraoui A, Barmeyer J, Muller KM (2000) Acute and chronic tissue response to coronary stent implantation: pathologic findings in human specimen. J Am Coll Cardiol 35:157–163
Palmerini T, Nedelman MA, Scudder LE et al (1999) Effects of abciximab on the acute pathology of blood vessels after arterial stenting in nonhuman primates (abstract). J Am Coll Cardiol 100:I–857
Simon DI, Xu H, Ortlepp S, Rogers C, Rao NK (1997) 7E3 monoclonal antibody directed against the platelet glycoprotein IIb/IIIa cross-reacts with the leukocyte integrin Mac-1 and blocks adhesion to fibrinogen and ICAM-1. Arterioscler Thromb Vasc Biol 17(3):528–535
Schwarz M, Nordt T, Bode C, Peter K (2002) The GP IIb/IIIa inhibitor abciximab (c7E3) inhibits the binding of various ligands to the leukocyte integrin Mac-1 (CD11b/CD18, alphaMbeta2). Thromb Res 107(3–4):121–128
Kupatt C, Habazettl H, Hanusch P, Wichels R, Hahnel D, Becker BF, Boekstegers P (2000) C7E3Fab reduces postischemic leukocyte-thrombocyte interaction mediated by fibrinogen. Implications for myocardial reperfusion injury. Arterioscler Thromb Vasc Biol 20(10):2226–2232
Byzova TV, Rabbani R, D’Souza SE, Plow EF (1998) Role of integrin alpha(v) beta3 in vascular biology. Thromb Haemost 80:726–734
Hoshiga M, Alpers CE Smith LL, Giachelli CM, Schwartz SM (1995) Alpha-v beta-3 integrin expression in normal and atherosclerotic artery. Circ Res 77:1129–1135
Stouffer GA, Hu Z, Sajid M et al (1998) Beta3 integrins are upregulated after vascular injury and modulate thrombospondin- and thrombin-induced proliferation of cultured smooth muscle cells. Circulation 97:907–915
Bendeck MP, Nakada MT (2001) The beta3 integrin antagonist m7E3 reduces matrix metalloproteinase activity and smooth muscle cell migration. J Vasc Res 38(6):590–599
Bishop GA, McPherson JA, Sanders J et al (1999) aVb3 receptor blockade reduces restenosis following balloon angioplasty in the atherosclerotic rabbit (abstract). J Am Coll Cardiol 33:31A
Julinda Mehilli, Adnan Kastrati, Helmut Schühlen et al (2004) Intracoronary stenting and antithrombotic regimen: is abciximab a superior way to eliminate elevated thrombotic risk in diabetics (isar-sweet) study investigators. Randomized clinical trial of abciximab in diabetic patients undergoing elective percutaneous coronary interventions after treatment with a high loading dose of clopidogrel Circulation 110:3627–3635
Gibson CM, Goel M, Cohen DJ et al (1998) Six-month angiographic and clinical follow-up of patients prospectively randomized to receive either tirofiban or placebo during angioplasty in the RESTORE trial. J Am Coll Cardiol 32:28–34
Tcheng JE, Kandzari DE, Grines CL, Cox DA, Effron MB, Garcia E, Griffin JJ, Guagliumi G, Stuckey T, Turco M, Fahy M, Lansky AJ, Mehran R, Stone GW (2003) CADILLAC Investigators. Benefits and risks of abciximab use in primary angioplasty for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. Circulation 108(11):1316–1323
Byzova TV, Plow EF (1998) Activation of alphaVbeta3 on vascular cells controls recognition of prothrombin. J Cell Biol 143:2081–2092
Thuraisingham S, Tan KH (1999) Dissolution of thrombus formed during direct coronary angioplasty with a single 10 mg intracoronary bolus dose of abciximab. Int J Clin Pract 53:604–607
Bailey SR, O’Leary E, Chilton R (1997) Angioscopic evaluation of site-specific administration of ReoPro. Cathet Cardiovasc Diagn 42:181–184
Bartorelli AL, Trabattoni D, Galli S, Grancini L, Cozzi S, Ravagnani P (1999) Successful dissolution of occlusive coronary thrombus with local administration of abciximab during PTCA. Catheter Cardiovasc Interv 48:211–213
Schlaifer JD, Horgan W, Malkowski MJ (2001) Acute thrombotic occlusion of the left main coronary artery in a hypercoagulable patient treated with intracoronary abciximab. Clin Cardiol 24:788
De Luca L, Bovenzi F, Signore N, D’Agostino C, de Luca I (2004) Primary angioplasty of an anomalous right coronary artery complicated by an acute thrombotic occlusion. Ital Heart J 5(10):785–788
Barsness GW, Buller C, Ohman EM et al (2000) Reduced thrombus burden with abciximab delivered locally before percutaneous intervention in saphenous vein grafts. Am Heart J 139:824–829
Wöhrle J, Grebe OC, Nusser T et al (2003) Reduction of major adverse cardiac events with intracoronary compared with intravenous bolus application of abciximab in patients with acute myocardial infarction or unstable angina undergoing coronary angioplasty. Circulation 107(14):1840–1843
Kakkar AK, Moustapha A, Hanley HG et al (2004) Comparison of intracoronary vs. intravenous administration of abciximab in coronary stenting. Catheter Cardiovasc Interv 61(1):31–34
Kintscher U, Kappert K, Schmidt G et al (2000) Effects of abciximab and tirofiban on vitronectin receptors in human endothelial and smooth muscle cells. Eur J Pharmacol 390:75–87
Bellandi F, Maioli M, Gallopin M, Toso A, Dabizzi RP (2004) Increase of myocardial salvage and left ventricular function recovery with intracoronary abciximab downstream of the coronary occlusion in patients with acute myocardial infarction treated with primary coronary intervention. Catheter Cardiovasc Interv 62(2):186–192
Prati F, Kwiatkowski P, Caroselli C, Imola F, Manzoli A, Fouad T, Corvo P, Ramazzotti V (2005) Use of abciximab prevents microcirculatory impairment in patients treated with coronary angioplasty for unstable angina: results of a prospective randomized study. Catheter Cardiovasc Interv 66:165–169
Marzilli M, Sambuceti G, Testa R, Fedele S (2002) Platelet glycoprotein IIb/IIIa receptor blockade and coronary resistance in unstable angina. J Am Coll Cardiol 40(12):2102–2109
Romagnoli E, Burzotta F, Trani C et al (2005) Angiographic evaluation of the effect of intracoronary Abciximab administration in patients undergoing urgent PCI. Int J Cardiol 105(3):250–255
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romagnoli, E., Burzotta, F., Trani, C. et al. Rationale for intracoronary administration of abciximab. J Thromb Thrombolysis 23, 57–63 (2007). https://doi.org/10.1007/s11239-006-9000-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-006-9000-0