Abstract
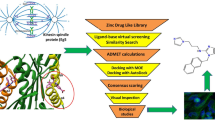
In an effort to contribute designing of improved anticancer molecules, in-silico prediction data including pharmacophore hypothesis, 3D-QSAR model, and molecular docking studies were performed on 55 compounds collected from literature. Tyrosine kinase has been used as a target enzyme as it plays a vital role in spleen cancer. The 3VF8 protein was selected for our molecular docking studies which is a crystal structure of spleen tyrosine kinase Syk catalytic domain with pyrazolylbenzimidazole inhibitor. A five-point pharmacophore was developed using 55 molecules having IC50 ranging from 13.2 to 0.83 μM. The best predictive pharmacophoric hypothesis ADRRR_2 was characterised by survival score 6.096, R2 = 0.8103, Q2 = 0.6169, F value = 20.3, RMSE = 0.45, Pearson-r = 0.4678, SD = 0.1905, and stability = 0.323 with a four-component PLS factor. In molecular docking studies, the compounds Bnz34, Bnz41, Bnz44, and Bnz45 showed interactions with MET448, LEU377, LEU307, LEU501, GLY378, LEU453, and LEU454 residues; these compounds have low-binding energies and a good in vivo activity. The pharmacophoric feature R5 is involved in hydrogen-bonding interaction whereas R7 is involved in aromatic hydrogen-bonding interactions with LEU377, GLY378, LEU501, LEU453, and LEU454 respectively. These results explained that one hydrogen acceptor, one hydrogen donor, and three aromatic rings are crucial for spleen tyrosine kinase inhibition.











Similar content being viewed by others
References
WHO (2016) The world cancer report from WHO, World Health Organization
Aydemir N, Bilaloglu R (2003) Genotoxicity of two anticancer drugs, gemcitabine and topotecan, in mouse bone marrow in vivo. Mutat Res 537:43–51
Brannon-Peppas L, Blanchette JO (2004) Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 56:821
Bari SB, Adhikari S, Surana SJ (2012) Tyrosine kinase receptor inhibitors: a new target for anticancer drug development. J Pharma SciTech 1(2):36–45
Riccaboni M, Bianchi I, Petrillo P (2010). Drug Discov Today 15(13–14)
Robert LG (2014) Getting Syk: spleen tyrosine kinase as a therapeutic target 35(8):414–422
SYK spleen associated tyrosine kinase [ Homo sapiens], Gene ID: 6850, 2017, NCBI
Nofali ZM, Soliman EA, El-Karimi SSA, Elzahari MI, Srouri AU, Sethumahavan S, Timothy J (2011) Novel benzimidazole derivatives as expected anticancer agents. Acta Poloniae Pharmaceutica ñ Drug Res 68(4):519–534
Kus C, Ayhan-Kilcigil G, Eke BC, Iscan M (2004) Synthesis and antioxidant properties of some novel benzimidazole derivatives on lipid peroxidation in the rat liver. Arch Pharm Res 27:156
Kumar JR, Jawahar JL, Pathak DP (2006) Synthesis of benzimidazole derivatives: as anti-hypertensive agents. E-J Chem 3:278–285
Kim JS, Sun Q, Yu C, Liu A, Liu LF, LaVoie EJ (1998) Quantitative structure-activity relationships on 5-substituted terbenzimidazoles as topoisomerase I poisons and antitumor agents. Bioorg Med Chem 6:163–172
Agh-Atabay NM, Dulger B, Gucin F (2003) Synthesis and investigation of antimicrobial activity of some bisbenzimidazole-derived chelating agents. Eur J Med Chem 38:875–881
Arjmand F, Mohani B, Ahmad S (2005) Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu(II) complex. Eur J Med Chem 40:1103–1110
Malaty H, El-Zimaity HMT, Gentra RM, Cole RA, Graham DY (1996) High-dose proton pump inhibitor plus amoxicillin for the treatment or retreatment of Helicobacter pylori infection. Aliment Pharm Therap 10(6):1001–1004
Matthews CJ, Broughton V, Bernardinelli G, Melich X, Brand G, Wills AC, Williams AF (2003) Molecular bricklaying: the protonated benzimidazole moiety as a synthon for crystal engineering. New J Chem 27:354–358
Roderick WR, Nordeen Jr CW, Von Esch AM, Appell RN (1972) Bisbenzimidazoles. Potent inhibitors of rhinoviruses. J Med Chem 15:655–658
Gunes HS, Cosar G (1992) Synthesis of some hydroxamic acid derivatives of benzimidazole and their antibacterial and antifungal activities. Arzneim-Forsch/Drug Res 42:1045–1048
Sun Q., Gatto B., Yu C., Liu A., Leroy L.F., La Voie E.J.,1994, Structure activity of topisomerase I poisons related to Hoechst 33342. Bioorg Med Chem, 4(24),2871–2876
Jin S, Kim JS, Sim S, Liu A, Pilch DS, Liu LF, La Voie EJ (2000) Heterocyclic bibenzimidazole derivatives as topoisomerase I ınhibitors. Bioorg Med ChemLett 10:719–723
Seaton A, Higgins C, Mann J, Baron A, Bailly C, Neidle S, Van den Berg H (2003) Mechanistic and anti-proliferative studies of two novel, biologically active bis-benzimidazoles. Eur J Cancer 39:2548–2555
Alpan AS, Gunes HS, Topcu Z (2007) 1H-Benzimidazole derivatives as mammalian DNA topoisomerase I inhibitors. ActaBiochim Pol 54(3):561–565
Göker H, Kus C, Boykin DW, Yildiz S, Altanlar N (2002) Synthesis of some new 2-substituted-phenyl-1H- benzimidazole-5-carbonitriles and their potent activity against Candida species. Biorg Med Chem 10:2589–2596
Ozden S, Atabey D, Yildiz S, Goker H (2005) Synthesis and potent antimicrobial activity of some novel ethyl or methyl 1-H-benzimidazole-5-carboxylates derivatives carrying amide or amidine group. Bioorg Med Chem 13:1587–1597
Nofal ZM, Fahmy HH, Mohamed HS (2002) Synthesis, antimicrobial and molluscicidal activities of new benzimidazole derivatives. Arch Pharm Res 25:250
Navarette-Vazquez G, Cedillo R, HernandezCampos A, Yepez L, Hernandez-Luis F, Valdez J, Morales R (2001) Synthesis and antiparasitic activity of 2-(trifluoromethyl)-benzimidazole derivatives. Bioorg Med Chem Lett 11:187
Soni JP, Dhrubo JS, Modh KM (2011) Structure activity relationship studies of pyrazolone derivative of imidazole, benzimidazole and benztriazole moiety of anti-inflammatory activity. Journal Appl Pharm Scie 1(4):115–120
Dixon SL, Smondyrev AM, Knoll EH, Rao SN, Shaw DE, Friesner RA (2006) PHASE: a new engine for pharmacophore perception, 3D QSAR model development, and 3D database screening: 1. Methodology and preliminary results. J Comput Aid Mol Des 20:647–671
Reddy TS, Kulhari H, Reddy VG, Bansal V, Ahmed K, Shukla R (2015) Design, synthesis and biological evaluation of 1,3-diphenyl-1H-pyrazole derivatives containing benzimidazole skeleton as potential anticancer and apoptosis inducing agents. Eur J Med Chem 101:790–805
Refaat HM (2010) Synthesis and anticancer activity of some novel 2-substituted benzimidazole derivatives. Eur J Med Chem 45:2949–2956
Shivakumar D, Williams J, Wu Y, Damm W, Shelley J, Sherman W (2010) Prediction of absolute salvation free energies using molecular dynamics free perturbation and OPLS force field. J Chem Theory Comput 6:1509–1519
Acknowledgements
We are also grateful to Dr. Pritesh and Mr. Vinod from Schrodinger for their expert assistance during the work and to Schrodinger for supplying us the software for this study.
Funding
RGUHS, Bangalore, Karnataka provided the financial assistance in a form of a research grant to carry out this work successfully.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dubey, S., Prabitha, P., Bhardwaj, S. et al. A study of pyrazolo-benzimidazole derivatives as spleen tyrosine kinase inhibitors: an in-silico approach. Struct Chem 30, 263–272 (2019). https://doi.org/10.1007/s11224-018-1189-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1189-y