Abstract
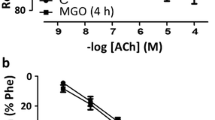
Postprandial hyperglycemia in diabetic and nondiabetic subjects is associated with endothelial dysfunction. Evidence shows that high glucose generates oxidative stress and a pro-inflammatory state promoting the development of cardiovascular diseases. trans-Resveratrol (t-RV) has been shown to reduce cardiovascular risk. To determine whether t-RV acts as a protector against acute high glucose (AHG)-induced damage, two in vitro models, rat aortic rings (RAR) and human umbilical vein endothelial cells (HUVEC) were used. RAR pretreated with AHG (25 mM D-glucose) for 3 h dramatically decreased the endothelium-dependent relaxation (EDR) induced by acetylcholine in phenylephrine (PE)-precontracted vessels. However, coincubation with t-RV significantly mitigated the damage induced by AHG on EDR. Pretreatment with AHG did not affect the vasodilation induced by sodium nitroprusside. HUVEC treated with t-RV decreased cytotoxicity and reduced radical oxygen species production induced by AHG. Taken together, these results suggest that t-RV can mitigate the AHG-induced EDR damage through a mechanism involving ROS scavenging and probably an increase in the bioavailability of NO.




Similar content being viewed by others
Abbreviations
- ACh:
-
Acetylcholine
- AHG:
-
Acute high glucose
- EC50 :
-
The concentration of the compound that gives half-maximal response
- EDR:
-
Endothelium-dependent relaxation
- Emax:
-
Emax maximal effect (relaxation)
- HUVEC:
-
Human umbilical vein endothelial cells
- KHB:
-
Krebs-Henseleit buffer
- MTS:
-
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- NO:
-
Nitric oxide
- PE:
-
Phenylephrine
- RAR:
-
Rat aortic rings
- ROS:
-
Reactive oxygen species
- SNP:
-
Sodium nitroprusside
- t-RV:
-
trans-Resveratrol
References
O'Keefe JH, Bell DS (2007) Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol 100:899–904
Fonseca V (2003) Clinical significance of targeting postprandial and fasting hyperglycemia in managing type 2 diabetes mellitus. Curr Med Res Opin 19:635–641
Colette C, Monnier L (2007) Acute glucose fluctuations and chronic sustained hyperglycemia as risk factors for cardiovascular diseases in patients with type 2 diabetes. Horm Metab Res 39:683–686
Mah E, Noh SK, Ballard KD et al (2011) Postprandial hyperglycemia impairs vascular endothelial function in healthy men by inducing lipid peroxidation and increasing asymmetric dimethylarginine:arginine. J Nutr 141:1961–1968
Loader J, Montero D, Lorenzen C, Watts R, Meziat C et al (2015) Acute hyperglycemia impairs vascular function in healthy and cardiometabolic diseased subjects: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol 35:2060–2072
Vorn R, Yoo HY (2017) Effects of high glucose with or without other metabolic substrates on alpha-adrenergic contractions in rat mesenteric and femoral arteries. Korean J Physiol Pharmacol 21:91–97
Tesfamariam B, Brown ML, Cohen RA (1991) Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest 87:1643–1648
Tesfamariam B, Cohen RA (1992) Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol Heart Circ Physiol 263:H321–H326
Tesfamariam B, Brown ML, Cohen RA (1992) Aldose reductase and myo-inositol in endothelial cell dysfunction caused by elevated glucose. J Pharmacol Exp Ther 263:153–157
Shuang-Xi W, Li-Ying L, Hu-Min Y-HL (2005) Na+/H+ exchanger inhibitor prevented endothelial dysfunction induced by high glucose. J Cardiovasc Pharmacol 45:586–590
Goel A, Zhang Y, Anderson L, Rahimian R (2007) Gender difference in rat aorta vasodilation after acute exposure to high glucose: involvement of protein kinase C beta and superoxide but not of Rho kinase. Cardiovasc Res 76:351–360
Meng XH, Ni C, Zhu L et al (2009) Puerarin protects against high glucose-induced acute vascular dysfunction: role of heme oxygenase-1 in rat thoracic aorta. Vasc Pharmacol 50:110–115
Vinet R, Martínez JL, Guzmán L (2015) The therapeutic potential of products based on polyphenols from wine grapes. In: Texeira-Duarte MC, Rai M (eds) Therapeutic medicinal plants: from lab to the market. CRC Press, New York, pp 331–342
Giovinazzo G, Grieco F (2015) Functional properties of grape and wine polyphenols. Plant Foods Hum Nutr 70:454–462
Khurana S, Venkataraman K, Hollingsworth A et al (2013) Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients 5:3779–3827
Orallo F, Alvarez E, Camiña M et al (2002) The possible implication of trans-resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol Pharmacol 61:294–302
Soylemez S, Gurdal H, Sepici A, Akar F (2008) The effect of long-term resveratrol treatment on relaxation to estrogen in aortae from male and female rats: role of nitric oxide and superoxide. Vasc Pharmacol 49:97–105
Develi-Is S, Ozen G, Bekpinar S et al (2014) Resveratrol improves high-fructose-induced vascular dysfunction in rats. Can J Physiol Pharmacol 92:1021–1027
Vinet R, Brieva C, Pinardi G, Penna M (1991) Influence of extracellular Ca2+ on the modulation of alpha-adrenergic-induced contractions in rat aorta. Gen Pharmacol 22:365–369
Wu D, Yotnda P (2011) Production and detection of reactive oxygen species (ROS) in cancers. J Vis Exp 57:e3357
Monnier L, Lapinski H, Colette C (2003) Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 26:881–885
Peter R, Dunseath G, Luzio SD, Chudleigh R, Choudhury SR, Owens DR (2009) Relative and absolute contributions of postprandial and fasting plasma glucose to daytime hyperglycaemia and HbA(1c) in subjects with type 2 diabetes. Diabet Med 26:974–980
Min Z, Kang L, Lin L et al (2010) Resveratrol restores lysophosphatidylcholine-induced loss of endothelium-dependent relaxation in rat aorta tissue coinciding with inhibition of extracellular-signal-regulated protein kinase activation. Phytother Res 24:1762–1768
Tang Y, Xu J, Qud W et al (2012) Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J Nutr Biochem 23:1410–1416
Sena CM, Pereira AM, Seiça R (2013) Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta 12:2216–2231
Matsumoto T, Kakami M, Noguchi E et al (2007) Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol 293:H1480–H1490
Mokhtar SS, Rasool AH (2015) Role of endothelium-dependent hyperpolarisation and prostacyclin in diabetes. Malays J Med Sci 22:8–17
Morrison KJ, Pollock D (1990) Impairment of relaxations to acetylcholine and nitric oxide by a phorbol ester in rat isolated aorta. Br J Pharmacol 101:432–436
Gordish KL, Beierwaltes WH (2014) Resveratrol induces acute endothelium-dependent renal vasodilation mediated through nitric oxide and reactive oxygen species scavenging. Am J Physiol Ren Physiol 306:F542–F550
Li T, Chen Y, Gua C, Li X (2017) Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front Physiol 8:350
Park SH, Jeong SO, Chung HT, Pae HO (2015) Pterostilbene, an active constituent of blueberries, stimulates nitric oxide production via activation of endothelial nitric oxide synthase in human umbilical vein endothelial cells. Plant Foods Hum Nutr 70:263–268
Lee KW, Kang NJ, Rogozin EA et al (2008) The resveratrol analogue 3,5,3,4,5-pentahydroxy-trans-stilbene inhibits cell transformation via MEK. Int J Cancer 123:2487–2496
Takizawa Y, Nakata R, Fukuhara K et al (2015) The 4′-hydroxyl group of resveratrol is functionally important for direct activation of PPARα. PLoS One 10:e0120865
Trachootham D, Lu W, Ogasawara MA et al (2008) Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374
Acknowledgements
This work was supported by Grants DIUV No. 57/2011 from Universidad de Valparaíso, Chile; Grant CREAS No. R12C1001 from CONICYT-REGIONAL, GORE Región de Valparaíso, Chile; Grant No. 037-274 from Pontificia Universidad Católica de Valparaíso, Chile and Grant DICYT-USACH No. 021643MS from JLM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors state no conflict of interest.
Rights and permissions
About this article
Cite this article
Guzmán, L., Balada, C., Flores, G. et al. t-Resveratrol Protects against Acute High Glucose Damage in Endothelial Cells. Plant Foods Hum Nutr 73, 235–240 (2018). https://doi.org/10.1007/s11130-018-0683-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-018-0683-0