Abstract
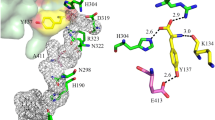
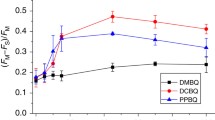
X-ray crystallographic analysis (1.9-Å resolution) of the cyanobacterial photosystem II (PSII) dimer showed the presence of five phosphatidylglycerol (PG) molecules per reaction center. One of the PG molecules, PG772, is located in the vicinity of the QB-binding site. To investigate the role of PG772 in PSII, we performed site-directed mutagenesis in the cytochrome (Cyt) b559 α subunit of Synechocystis sp. PCC 6803 to change two amino acids, Thr-5 and Ser-11, which interact with PG772. The photosynthetic activity of intact cells was slightly lower in all mutants than that of cells in the control strain; however, the oxygen-evolving PSII activity was decreased markedly in cells of mutants, as measured using artificial quinones (such as p-benzoquinone). Furthermore, electron transport from QA to QB was inhibited in mutants incubated with quinones, particularly under high-intensity light conditions. Lipid analysis of purified PSII showed approximately one PG molecule per reaction center, presumably PG772, was lost in the PSII dimer from the T5A and S11A mutants compared with that in the PSII dimer from the control strain. In addition, protein analysis of monomer and dimer showed decreased levels of PsbV and PsbU extrinsic proteins in the PSII monomer purified from T5A and S11A mutants. These results suggest that site-directed mutagenesis of Thr-5 and Ser-11, which presumably causes the loss of PG772, induces quinone-dependent inhibition of PSII activity under high-intensity light conditions and destabilizes the binding of extrinsic proteins to PSII.







Similar content being viewed by others
Abbreviations
- BQ:
-
p-Benzoquinone
- BN:
-
Blue native
- Chl:
-
Chlorophyll
- Cyt:
-
Cytochrome
- DCBQ:
-
2,6-Dichloro-p-benzoquinone
- DCMU:
-
3-(3,4-Dichlorophenyl)-1,1-dimethylurea
- DGDG:
-
Digalactosyldiacylglycerol
- DM:
-
n-Dodecyl-β-d-maltoside
- DMBQ:
-
2,6-Dimethyl-p-benzoquinone
- Em:
-
Erythromycin
- EmR :
-
Erythromycin-resistant gene cassette
- Fecy:
-
Potassium ferricyanide
- HP:
-
High potential
- IP:
-
Intermediate potential
- Km:
-
Kanamycin
- LP:
-
Low potential
- MGDG:
-
Monogalactosyldiacylglycerol
- PG:
-
Phosphatidylglycerol
- PQ:
-
Plastoquinone
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SQDG:
-
Sulfoquinovosyldiacylglycerol
References
Barbato R, Friso G, Ponticos M, Barber J (1995) Characterization of the light-induced cross-linking of the α-subunit of cytochrome b 559 and the D1 protein in isolated photosystem II reaction centers. J Biol Chem 270:24032–24037
Bligh EG, Dyer WJ (1961) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Chiu YF, Lin WC, Wu CM, Chen YH, Hung CH, Ke SC, Chu HA (2009) Identification and characterization of a cytochrome b 559 Synechocystis 6803 mutant spontaneously generated from DCMU-inhibited photoheterotrophical growth conditions. Biochim Biophys Acta 1787:1179–1188
Chiu YF, Chen YH, Roncel M, Dilbeck PL, Huang JY, Ke SC, Ortega JM, Burnap RL, Chu HA (2013) Spectroscopic and functional characterization of cyanobacterium Synechocystis PCC 6803 mutants on the cytoplasmic-side of cytochrome b 559 in photosystem II. Biochim Biophys Acta 1827:507–519
Chu HA, Chiu YF (2016) The roles of cytochrome b 559 in assembly and photoprotection of photosystem II revealed by site-directed mutagenesis studies. Front Plant Sci 6:1–7
Endo K, Mizusawa N, Shen JR, Yamada M, Tomo T, Komatsu H, Kobayashi M, Kobayashi K, Wada H (2015) Site-directed mutagenesis of amino acid residues of D1 protein interacting with phosphatidylglycerol affects the function of plastoquinone QB in photosystem II. Photosynth Res 126:385–397
Endo K, Kobayashi K, Wada H (2016) Sulfoquinovosyldiacylglycerol has an essential role in Thermosynechococcus elongatus BP-1 under phosphate-deficient conditions. Plant Cell Physiol 57:2461–2471
Gombos Z, Wada H, Murata N (1991) Direct evaluation of effects of fatty-acid unsaturation on the thermal-properties of photosynthetic activities, as studied by mutation and transformation of Synechocystis PCC 6803. Plant Cell Physiol 32:205–211
Gombos Z, Várkonyi Z, Hagio M, Iwaki M, Kovács L, Masamoto K, Itoh S, Wada H (2002) Phosphatidylglycerol requirement for the function of electron acceptor plastoquinone QB in the photosystem II reaction center. Biochemistry 41:3796–3802
Guskov A, Kern J, Gabdulkhakov A, Broser M, Zouni A, Saenger W (2009) Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nat Struct Mol Biol 16:334–342
Hagio M, Gombos Z, Várkonyi Z, Masamoto K, Sato N, Tsuzuki M, Wada H (2000) Direct evidence for requirement of phosphatidylglycerol in photosystem II of photosynthesis. Plant Physiol 124:795–804
Hung CH, Huang JY, Chiu YF, Chu HA (2007) Site-directed mutagenesis on the heme axial-ligands of cytochrome b 559 in photosystem II by using cyanobacteria Synechocystis PCC 6803. Biochim Biophys Acta 1767:686–693
Hung CH, Hwang HJ, Chen YH, Chiu YF, Ke SC, Burnap RL, Chu HA (2010) Spectroscopic and functional characterizations of cyanobacterium Synechocystis PCC 6803 mutants on and near the heme axial ligand of cytochrome b 559 in photosystem II. J Biol Chem 285:5653–5663
Itoh S, Kozuki T, Nishida K, Fukushima Y, Yamakawa H, Domonkos I, Laczkó-Dobos H, Kis M, Ughy B, Gombos Z (2012) Two functional sites of phosphatidylglycerol for regulation of reaction of plastoquinone QB in photosystem II. Biochim Biophys Acta 1817:287–297
Kaminskaya O, Shuvalov VA, Renger G (2007a) Evidence for a novel quinone-binding site in the photosystem II (PS II) complex that regulates the redox potential of cytochrome b 559. Biochemistry 46:1091–1105
Kaminskaya O, Shuvalov VA, Renger G (2007b) Two reaction pathways for transformation of high potential cytochrome b 559 of PS II into the intermediate potential form. Biochim Biophys Acta 1767:550–558
Kashino Y, Lauber WM, Carroll JA, Wang Q, Whitmarsh J, Satoh K, Pakrasi HB (2002) Proteomic analysis of a highly active photosystem II preparation from the cyanobacterium Synechocystis sp. PCC 6803 reveals the presence of novel polypeptides. Biochemistry 41:8004–8012
Kern J, Renger G (2007) Photosystem II: structure and mechanism of the water: plastoquinone oxidoreductase. Photosynth Res 94:183–202
Kobayashi K, Endo K, Wada H (2016) Roles of lipids in photosynthesis. In: Nakamura Y, Li-Beisson Y (eds) Lipids in plant and algae development. Springer International Publishing, Cham, pp. 21–49
Kopečná J, Pilný J, Krynická V, Tomčala A, Kis M, Gombos Z, Komenda J, Sobotka R (2015) Lack of phosphatidylglycerol inhibits chlorophyll biosynthesis at multiple sites and limits chlorophyllide reutilization in Synechocystis sp. strain PCC 6803. Plant Physiol 169:1307–1317
Krieger-Liszkay A, Rutherford AW (1998) Influence of herbicide binding on the redox potential of the quinone acceptor in photosystem II: Relevance to photodamage and phytotoxicity. Biochemistry 37:17339–17344
Lupínková L, Metz JG, Diner BA, Vass I, Komenda J (2002) Histidine residue 252 of the photosystem II D1 polypeptide is involved in a light-induced cross-linking of the polypeptide with the α subunit of cytochrome b-559: study of a site-directed mutant of Synechocystis PCC 6803. Biochim Biophys Acta 1554:192–201
Mizusawa N, Wada H (2012) The role of lipids in photosystem II. Biochim Biophys Acta 1817:194–208
Morita SY, Terada T (2015) Enzymatic measurement of phosphatidylglycerol and cardiolipin in cultured cells and mitochondria. Sci Rep 5:1–15
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421
Nishiyama Y, Allakhverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta 1757:742–749
Pagliano C, Saracco G, Barber J (2013) Structural, functional and auxiliary proteins of photosystem II. Photosynth Res 116:16–188
Pakrasi HB, Williams JG, Arntzen CJ (1988) Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: evidence for a functional role of cytochrome b 559 in photosystem II. EMBO J 7:325–332
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Renger G, Renger T (2008) Photosystem II: the machinery of photosynthetic water splitting. Photosynth Res 98:53–80
Roncel M, Ortega JM, Losada M (2001) Factors determining the special redox properties of photosynthetic cytochrome b 559. Eur J Biochem 268:4961–4968
Sakurai I, Hagio M, Gombos Z, Tyystjarvi T, Paakkarinen V, Aro EM, Wada H (2003) Requirement of phosphatidylglycerol for maintenance of photosynthetic machinery. Plant Physiol 133:1376–1384
Sakurai I, Mizusawa N, Ohashi S, Kobayashi M, Wada H (2007a) Effects of the lack of phosphatidylglycerol on the donor side of photosystem II. Plant Physiol 144:1336–1346
Sakurai I, Mizusawa N, Wada H, Sato N (2007b) Digalactosyldiacylglycerol is required for stabilization of the oxygen-evolving complex in photosystem II. Plant Physiol 145:1361–1370
Sato N, Hagio M, Wada H, Tsuzuki M (2000) Requirement of phosphatidylglycerol for photosynthetic function in thylakoid membranes. Proc Natl Acad Sci USA 97:10655–10660
Satoh K, Koike H, Ichimura T, Katoh S (1992) Binding affinities of benzoquinones to the QB site of photosystem II in Synechococcus oxygen-evolving preparation. Biochim Biophys Acta 1102:45–52
Satoh K, Oh-hashi M, Kashino Y, Koike H (1995) Mechanism of electron flow through the QB site in photosystem II. 1. Kinetics of the reduction of electron acceptors at the QB and plastoquinone sites in photosystem II particles from the cyanobacterium Synechococcus vulcanus. Plant Cell Physiol 36:597–605
Shi LX, Hall M, Funk C, Schröder WP (2012) Photosystem II, a growing complex: updates on newly discovered components and low molecular mass proteins. Biochim Biophys Acta 1817:13–25
Shinopoulos KE, Brudvig GW (2012) Cytochrome b 559 and cyclic electron transfer within photosystem II. Biochim Biophys Acta 1817:66–75
Stewart DH, Brudvig GW (1998) Cytochrome b 559 of photosystem II. Biochim Biophys Acta 1367:63–87
Tyystjärvi E (2008) Photoinhibition of photosystem II and photodamage of the oxygen evolving manganese cluster. Coord Chem Rev 252:361–376
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60
Van Eerden FJ, Melo MN, Frederix PWJM, Periole X, Marrink SJ (2017) Exchange pathways of plastoquinone and plastoquinol in the photosystem II complex. Nat Commun 8:15214
Acknowledgements
This work was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (16J10129), and by CREST, Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
BN-PAGE/SDS-PAGE analyses of PSII complexes purified from cells of the control strain and mutants. PSII complexes corresponding to 3 μg Chl were analyzed by BN-PAGE in the first dimension, and then by SDS-PAGE in the second dimension. Proteins in the gels of SDS-PAGE were visualized with silver staining. (TIF 6142 KB)
Rights and permissions
About this article
Cite this article
Endo, K., Kobayashi, K., Wang, HT. et al. Site-directed mutagenesis of two amino acid residues in cytochrome b559 α subunit that interact with a phosphatidylglycerol molecule (PG772) induces quinone-dependent inhibition of photosystem II activity. Photosynth Res 139, 267–279 (2019). https://doi.org/10.1007/s11120-018-0555-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-018-0555-3