Abstract
Purpose
The objective was to develop, characterize and assess the potentiality of W1/O/W2 self-emulsifying multiple nanoemulsions to enhance signal/noise ratio for Magnetic Resonance Imaging (MRI).
Methods
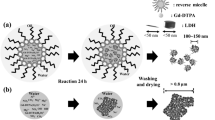
For this purpose, a new formulation, was designed for encapsulation efficiency and stability. Various methods were used to characterize encapsulation efficiency ,in particular calorimetric methods (Differential Scanning Calorimetry (DSC), thermogravimetry analysis) and ultrafiltration. MRI in vitro relaxivities were assessed on loaded DTPA-Gd multiple nanoemulsions.
Results
Characterization of the formulation, in particular of encapsulation efficiency was a challenge due to interactions found with ultrafiltration method. Thanks to the specifically developed DSC protocol, we were able to confirm the formation of multiple nanoemulsions, differentiate loaded from unloaded nanoemulsions and measure the encapsulation efficiency which was found to be quite high with a 68% of drug loaded. Relaxivity studies showed that the self-emulsifying W/O/W nanoemulsions were positive contrast agents, exhibiting higher relaxivities than those of the DTPA-Gd solution taken as a reference.
Conclusion
New self-emulsifying multiple nanoemulsions that were able to load satisfactory amounts of contrasting agent were successfully developed as potential MRI contrasting agents. A specific DSC protocol was needed to be developed to characterize these complex systems as it would be useful to develop these self-formation formulations.





Similar content being viewed by others
Abbreviations
- DLS:
-
Dynamic Light Scattering
- DSC:
-
Differential Scanning Calorimetry
- DTPA-Eu:
-
Diethylene tri-amine penta acetic acid—Europium
- DTPA-Gd:
-
Diethylene tri-amine penta acetic acid—Gadolinium
- E.E:
-
Encapsulation efficiency
- HSA:
-
Human Serum Albumin
- ICP-AES:
-
Inductively Coupled Plasma Absorption Emission Spectroscopy
- ICP-MS:
-
Inductively Coupled Plasma Mass Spectrometry
- MDS:
-
Mean Droplet Size
- MRI:
-
Magnetic Resonance Imaging
- PDI:
-
Polydispersity Index
- TEM:
-
Transmission Electron Microscopy
- TGA:
-
Thermo Gravimetric Analysis
References
Pays K, Giermanska-Kahn J, Pouligny B, Bibette J, Leal-Calderon F. Double emulsions: how does release occur? J Control Release. 2002;79:193–205.
Doiron AL, Chu K, Ali A, Brannon-Peppas L. Preparation and initial characterization of biodegradable particles containing gadolinium-DTPA contrast agent for enhanced MRI. Proc Natl Acad Sci U S A. 2008;105:17232–7.
Sigward E, Mignet N, Rat P, Dutot M, Muhamed S, Guigner JM, et al. Formulation and cytotoxicity evaluation of new self-emulsifying multiple W/O/W nanoemulsions. Int J Nanomed. 2013;8:611–25.
Gupta S. Biocompatible microemulsion systems for drug encapsulation and delivery. Curr Sci. 2011;101:174–88.
Chuan YP, Zeng BY, O’Sullivan B, Thomas R, Middelberg APJ. Co-delivery of antigen and a lipophilic anti-inflammatory drug to cells via a tailorable nanocarrier emulsion. J Colloid Interface Sci. 2012;368:616–24.
Gianella A, Jarzyna PA, Mani V, Ramachandran S, Calcagno C, Tang J, et al. Multifunctional Nanoemulsion platform for imaging guided therapy evaluated in experimental cancer. ACS Nano. 2011;5:4422–33.
Pouton CW. Formulation of self-emulsifying drug delivery systems. Adv Drug Deliv Rev. 1997;25:47–58.
Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10:102–10.
Gao GH, Lee JW, Nguyen MK, Im GH, Yang J, Heo H, et al. pH-responsive polymeric micelle based on PEG-poly(β-amino ester)/(amido amine) as intelligent vehicle for magnetic resonance imaging in detection of cerebral ischemic area. J Control Release. 2011;155:11–7.
Nakamura E, Makino K, Okano T, Yamamoto T, Yokoyama M. A polymeric micelle MRI contrast agent with changeable relaxivity. J Control Release. 2006;114:325–33.
Mulder WJM, Strijkers GJ, van Tilborg GAF, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006;19:142–64.
Hak S, Sanders HMHF, Agrawal P, Langereis S, Grüll H, Keizer HM, et al. A high relaxivity Gd(III)DOTA-DSPE-based liposomal contrast agent for magnetic resonance imaging. Eur J Pharm Biopharm. 2009;72:397–404.
Mulder WJM, Strijkers GJ, Griffioen AW, van Bloois L, Molema G, Storm G, et al. A liposomal system for contrast-enhanced magnetic resonance imaging of molecular targets. Bioconjug Chem. 2004;15:799–806.
Luciani A, Olivier J-C, Clement O, Siauve N, Brillet P-Y, Bessoud B, et al. Glucose-receptor MR imaging of tumors: study in mice with PEGylated paramagnetic niosomes. Radiology. 2004;231:135–42.
Sainsbury F, Zeng B, Middelberg APJ. Towards designer nanoemulsions for precision delivery of therapeutics. Curr Opin Chem Eng. 2014;4:11–7.
Yang F, Gu A, Chen Z, Gu N, Ji M. Multiple emulsion microbubbles for ultrasound imaging. Mater Lett. 2008;62:121–4.
Jarzyna PA, Skajaa T, Gianella A, Cormode DP, Samber DD, Dickson SD, et al. Iron oxyde core oil-in-water emulsions as a multifonctional nanoparticle platform for tumor targeting and imaging. Biomaterials. 2009;30:6947–54.
Li C, Liu Q, Mei Z, Wang J, Xu J, Sun D. Pickering emulsions stabilized by paraffin wax and Laponite clay particles. J Colloid Interface Sci. 2009;336:314–21.
Nepal PR, Han H-K, Choi H-K. Preparation and in vitro–in vivo evaluation of Witepsol® H35 based self-nanoemulsifying drug delivery systems (SNEDDS) of coenzyme Q10. Eur J Pharm Sci. 2010;39:224–32.
Bouyer E, Mekhloufi G, Rosilio V, Grossiord J-L, Agnely F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: alternatives to synthetic surfactants in the pharmaceutical field? Int J Pharm. 2012;436:359–78.
Schuch A, Köhler K, Schuchmann HP. Differential scanning calorimetry (DSC) in multiple W/O/W emulsions. J Therm Anal Calorim. 2013;111:1881–90.
Bos MA, van Vliet T. Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv Colloid Interf Sci. 2001;91:437–71.
Damodaran S. Protein stabilization of emulsions and foams. J Food Sci. 2005;70:R54–66.
Elster AD, Jackels SC, Allen NS, Marrache RC. Dyke award. Europium-DTPA: a gadolinium analogue traceable by fluorescence microscopy. Am J Neuroradiol. 1989;10:1137–44.
Mignet N, Chermont Q, Randrianarivelo T, Seguin J, Richard C, Bessodes M, et al. Liposome biodistribution by time resolved fluorimetry of lipophilic europium complexes. Eur Biophys J. 2006;35:155–61.
Gan L, Gan Y, Zhu C, Zhang X, Zhu J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: in vitro and in vivo results. Int J Pharm. 2009;365:143–9.
Clausse D. Thermal behaviour of emulsions studied by differential scanning calorimetry. J Therm Anal Calorim. 1998;51:191–201.
Leyendekkers JV, Hunter RJ. Thermodynamic properties of water in the subcooled region. I. J Chem Phys. 1985;82:1440–6.
Schuch A, Deiters P, Henne J, Köhler K, Schuchmann HP. Production of W/O/W (water-in-oil-in-water) multiple emulsions: droplet breakup and release of water. J Colloid Interface Sci. 2013;402:157–64.
Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev. 2008;60:625–37.
Delmas T. How to prepare and stabilize very small nanoemulsions. Langmuir. 2011;27:1683–92.
Clausse D, Gomez F, Pezron I, Komunjer L, Dalmazzone C. Morphology characterization of emulsions by differential scanning calorimetry. Adv Colloid Interf Sci. 2005;117:59–74.
Mezzenga R, Folmer BM, Hughes E. Design of double emulsions by osmotic pressure tailoring. Langmuir. 2004;20:3574–82.
Chou DK, Krishnamurthy R, Randolph TW, Carpenter JF, Manning MC. Effects of Tween 20® and Tween 80® on the stability of Albutropin during agitation. J Pharm Sci. 2005;94:1368–81.
Laurent S, Henoumont C, Vander Elst L, Muller RN. Synthesis and physicochemical characterisation of Gd-DTPA derivatives as contrast agents for MRI.Eur J. Inorg Chem. 2012;12:1889–915.
Tilcock C, Unger E, Cullis P, MacDougall P. Liposomal Gd-DTPA: preparation and characterization of relaxivity. Radiology. 1989;171:77–80.
Unger E, Shen DK, Wu G, Fritz T. Liposomes as MR contrast agents: pros and cons. Magn Reson Med. 1991;22:304–8.
Fossheim SL, Fahlvik AK, Klaveness J, Muller RN. Paramagnetic liposomes as MRI contrast agents: influence of liposomal physicochemical properties on the in vitro relaxivity. Magn Reson Imaging. 1999;17:83–9.
Strijkers GJ, Mulder WJM, van Heeswijk RB, Frederik PM, Bomans P, Magusin PCMM, et al. Relaxivity of liposomal paramagnetic MRI contrast agents. Magn Reson Mater Phys Biol Med. 2005;18:186–92.
Dekker M. In: Cevc G, editor. Phospholipids handbook. New York: CRC Press; 1993. p. 988.
Magdassi S, Garti N. Release of electrolytes in multiple emulsions: coalescence and breakdown or diffusion through oil phase? Colloids Surf. 1984;12:367–73.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors wish to thank Jean-Michel Guigner, Institut de minéralogie et de physique des milieu condensés IMPC-IRD-CNRS UMR 7590, UPMC for his support for TEM imaging and ED387-iViV, UPMC Sorbonne Université, Paris, France for supporting this project.
We thank the Maison des Langues, Université Paris Descartes, Sorbonne Paris Cité, 45 rue des Saints-Pères, 75006 Paris France for their review of the English manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sigward, E., Corvis, Y., Doan, BT. et al. Preparation and Evaluation of Multiple Nanoemulsions Containing Gadolinium (III) Chelate as a Potential Magnetic Resonance Imaging (MRI) Contrast Agent. Pharm Res 32, 2983–2994 (2015). https://doi.org/10.1007/s11095-015-1680-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1680-8