Abstract
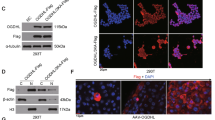
The antioxidant glutathione (GSH) plays a critical role in maintaining intracellular redox homeostasis but in tumors the GSH biosynthetic pathway is often dysregulated, contributing to tumor resistance to radiation and chemotherapy. Glutamate-cysteine ligase (GCL) catalyzes the first and rate-limiting reaction in GSH synthesis, and enzyme function is controlled by GSH feedback inhibition or by transcriptional upregulation of the catalytic (GCLC) and modifier (GCLM) subunits. However, it has recently been reported that the activity of GCLC and the formation of GCL can be modified by reactive aldehyde products derived from lipid peroxidation. Due to the susceptibility of GCLC to posttranslational modifications by reactive aldehydes, we examined the potential for 2-deoxy-d-ribose (2dDR) to glycate GCLC and regulate enzyme activity and GCL formation. 2dDR was found to directly modify both GCLC and GCLM in vitro, resulting in a significant inhibition of GCLC and GCL enzyme activity without altering substrate affinity or feedback inhibition. 2dDR-mediated glycation also inhibited GCL subunit heterodimerization and formation of the GCL holoenzyme complex while not causing dissociation of pre-formed holoenzyme. This PTM could be of particular importance in glioblastoma (GBM) where intratumoral necrosis provides an abundance of thymidine, which can be metabolized by thymidine phosphorylase (TP) to form 2dDR. TP is expressed at high levels in human GBM tumors and shRNA knockdown of TP in U87 GBM cells results in a significant increase in cellular GCL enzymatic activity.







Similar content being viewed by others
References
Meloche J, Paulin R, Courboulin A, Lambert C, Barrier M, Bonnet P, Bisserier M, Roy M, Sussman MA, Agharazii M, Bonnet S (2011) RAGE-dependent activation of the oncoprotein Pim1 plays a critical role in systemic vascular remodeling processes. Arterioscler Thromb Vasc Biol 31(9):2114–2124. doi:10.1161/ATVBAHA.111.230573
Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT (2009) RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 7:17. doi:10.1186/1479-5876-7-17
Singh R, Barden A, Mori T, Beilin L (2001) Advanced glycation end-products: a review. Diabetologia 44(2):129–146. doi:10.1007/s001250051591
Goldin A, Beckman JA, Schmidt AM, Creager MA (2006) Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114(6):597–605. doi:10.1161/CIRCULATIONAHA.106.621854
Cho SJ, Roman G, Yeboah F, Konishi Y (2007) The road to advanced glycation end products: a mechanistic perspective. Curr Med Chem 14(15):1653–1671
Watkins NG, Thorpe SR, Baynes JW (1985) Glycation of amino groups in protein. Studies on the specificity of modification of RNase by glucose. J Biol Chem 260(19):10629–10636
Horiuchi S (1996) Advanced glycation end products (AGE)-modified proteins and their potential relevance to atherosclerosis. Trends Cardiovasc Med 6(5):163–168. doi:10.1016/1050-1738(96)00050-3
Syrovy I (1994) Glycation of albumin: reaction with glucose, fructose, galactose, ribose or glyceraldehyde measured using four methods. J Biochem Biophys Methods 28(2):115–121
Khalifah RG, Todd P, Booth AA, Yang SX, Mott JD, Hudson BG (1996) Kinetics of nonenzymatic glycation of ribonuclease A leading to advanced glycation end products. Paradoxical inhibition by ribose leads to facile isolation of protein intermediate for rapid post-Amadori studies. Biochemistry 35(15):4645–4654. doi:10.1021/bi9525942
Brown NS, Bicknell R (1998) Thymidine phosphorylase, 2-deoxy-d-ribose and angiogenesis. Biochem J 334(Pt 1):1–8
Brown NS, Jones A, Fujiyama C, Harris AL, Bicknell R (2000) Thymidine phosphorylase induces carcinoma cell oxidative stress and promotes secretion of angiogenic factors. Cancer Res 60(22):6298–6302
de Bruin M, Smid K, Laan AC, Noordhuis P, Fukushima M, Hoekman K, Pinedo HM, Peters GJ (2003) Rapid disappearance of deoxyribose-1-phosphate in platelet derived endothelial cell growth factor/thymidine phosphorylase overexpressing cells. Biochem Biophys Res Commun 301(3):675–679
Takino J, Yamagishi S, Takeuchi M (2012) Glycer-AGEs-RAGE signaling enhances the angiogenic potential of hepatocellular carcinoma by upregulating VEGF expression. World J Gastroenterol 18(15):1781–1788. doi:10.3748/wjg.v18.i15.1781
Rojas A, Gonzalez I, Morales E, Perez-Castro R, Romero J, Figueroa H (2011) Diabetes and cancer: looking at the multiligand/RAGE axis. World J Diabetes 2(7):108–113. doi:10.4239/wjd.v2.i7.108
Bijnsdorp IV, Capriotti F, Kruyt FA, Losekoot N, Fukushima M, Griffioen AW, Thijssen VL, Peters GJ (2011) Thymidine phosphorylase in cancer cells stimulates human endothelial cell migration and invasion by the secretion of angiogenic factors. Br J Cancer 104(7):1185–1192. doi:10.1038/bjc.2011.74
Takebayashi Y, Yamada K, Miyadera K, Sumizawa T, Furukawa T, Kinoshita F, Aoki D, Okumura H, Yamada Y, Akiyama S, Aikou T (1996) The activity and expression of thymidine phosphorylase in human solid tumours. Eur J Cancer 32A(7):1227–1232
Hirano H, Tanioka K, Yokoyama S, Akiyama S, Kuratsu J (2001) Angiogenic effect of thymidine phosphorylase on macrophages in glioblastoma multiforme. J Neurosurg 95(1):89–95. doi:10.3171/jns.2001.95.1.0089
Takano S, Tsuboi K, Matsumura A, Tomono Y, Mitsui Y, Nose T (2000) Expression of the angiogenic factor thymidine phosphorylase in human astrocytic tumors. J Cancer Res Clin Oncol 126(3):145–152
Tanioka K, Takeshima H, Hirano H, Kimura T, Nagata S, Akiyama S, Kuratsu J (2001) Biological role of thymidine phosphorylase in human astrocytic tumors. Oncol Rep 8(3):491–496
Yao Y, Kubota T, Sato K, Kitai R (2001) Macrophage infiltration-associated thymidine phosphorylase expression correlates with increased microvessel density and poor prognosis in astrocytic tumors. Clin Cancer Res 7(12):4021–4026
Noch E, Khalili K (2009) Molecular mechanisms of necrosis in glioblastoma: the role of glutamate excitotoxicity. Cancer Biol Ther 8(19):1791–1797
Raza SM, Fuller GN, Rhee CH, Huang S, Hess K, Zhang W, Sawaya R (2004) Identification of necrosis-associated genes in glioblastoma by cDNA microarray analysis. Clin Cancer Res 10(1 Pt 1):212–221
Miyadera K, Sumizawa T, Haraguchi M, Yoshida H, Konstanty W, Yamada Y, Akiyama S (1995) Role of thymidine phosphorylase activity in the angiogenic effect of platelet derived endothelial cell growth factor/thymidine phosphorylase. Cancer Res 55(8):1687–1690
Griffiths L, Stratford IJ (1997) Platelet-derived endothelial cell growth factor thymidine phosphorylase in tumour growth and response to therapy. Br J Cancer 76(6):689–693
Nakajima Y, Madhyastha R, Maruyama M (2009) 2-Deoxy-d-ribose, a downstream mediator of thymidine phosphorylase, regulates tumor angiogenesis and progression. Anticancer Agents Med Chem 9(2):239–245
Ikeda R, Furukawa T, Kitazono M, Ishitsuka K, Okumura H, Tani A, Sumizawa T, Haraguchi M, Komatsu M, Uchimiya H, Ren XQ, Motoya T, Yamada K, Akiyama S (2002) Molecular basis for the inhibition of hypoxia-induced apoptosis by 2-deoxy-d-ribose. Biochem Biophys Res Commun 291(4):806–812. doi:10.1006/bbrc.2002.6432
Moghaddam A, Zhang HT, Fan TP, Hu DE, Lees VC, Turley H, Fox SB, Gatter KC, Harris AL, Bicknell R (1995) Thymidine phosphorylase is angiogenic and promotes tumor growth. Proc Natl Acad Sci USA 92(4):998–1002
Schmidt MM, Greb H, Koliwer-Brandl H, Kelm S, Dringen R (2010) 2-deoxyribose deprives cultured astrocytes of their glutathione. Neurochem Res 35(11):1848–1856. doi:10.1007/s11064-010-0251-y
Fico A, Manganelli G, Cigliano L, Bergamo P, Abrescia P, Franceschi C, Martini G, Filosa S (2008) 2-deoxy-d-ribose induces apoptosis by inhibiting the synthesis and increasing the efflux of glutathione. Free Radic Biol Med 45(2):211–217. doi:10.1016/j.freeradbiomed.2008.04.017
Ardestani A, Yazdanparast R, Nejad AS (2008) 2-Deoxy-d-ribose-induced oxidative stress causes apoptosis in human monocytic cells: prevention by pyridoxal-5′-phosphate. Toxicol In Vitro 22(4):968–979. doi:10.1016/j.tiv.2008.02.010
Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ (2009) Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med 30(1–2):86–98
Ramamurthy B, Jones AD, Larsson L (2003) Glutathione reverses early effects of glycation on myosin function. Am J Physiol Cell Physiol 285(2):C419–C424. doi:10.1152/ajpcell.00502.2002
Ajiboye R, Harding JJ (1989) The non-enzymic glycosylation of bovine lens proteins by glucosamine and its inhibition by aspirin, ibuprofen and glutathione. Exp Eye Res 49(1):31–41
Jain SK (1998) Glutathione and glucose-6-phosphate dehydrogenase deficiency can increase protein glycosylation. Free Radic Biol Med 24(1):197–201
Backos DS, Franklin CC, Reigan P (2012) The role of glutathione in brain tumor drug resistance. Biochem Pharmacol 83(8):1005–1012. doi:10.1016/j.bcp.2011.11.016
Lu SC (2009) Regulation of glutathione synthesis. Mol Aspects Med 30(1–2):42–59
Griffith OW, Mulcahy RT (1999) The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Adv Enzymol Relat Areas Mol Biol 73:209–267
Yang Y, Chen Y, Johansson E, Schneider SN, Shertzer HG, Nebert DW, Dalton TP (2007) Interaction between the catalytic and modifier subunits of glutamate-cysteine ligase. Biochem Pharmacol 74(2):372–381. doi:10.1016/j.bcp.2007.02.003
Tu Z, Anders MW (1998) Expression and characterization of human glutamate-cysteine ligase. Arch Biochem Biophys 354(2):247–254
Backos DS, Fritz KS, Roede JR, Petersen DR, Franklin CC (2011) Posttranslational modification and regulation of glutamate-cysteine ligase by the alpha, beta-unsaturated aldehyde 4-hydroxy-2-nonenal. Free Radic Biol Med 50(1):14–26. doi:10.1016/j.freeradbiomed.2010.10.694c
Krzywanski DM, Dickinson DA, Iles KE, Wigley AF, Franklin CC, Liu RM, Kavanagh TJ, Forman HJ (2004) Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch Biochem Biophys 423(1):116–125
Backos DS, Brocker CN, Franklin CC (2010) Manipulation of cellular GSH biosynthetic capacity via TAT-mediated protein transduction of wild-type or a dominant-negative mutant of glutamate cysteine ligase alters cell sensitivity to oxidant-induced cytotoxicity. Toxicol Appl Pharmacol 243(1):35–45. doi:10.1016/j.taap.2009.11.010
White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ (2003) Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal Biochem 318(2):175–180
Munanairi A, O’Banion SK, Gamble R, Breuer E, Harris AW, Sandwick RK (2007) The multiple Maillard reactions of ribose and deoxyribose sugars and sugar phosphates. Carbohydr Res 342(17):2575–2592. doi:10.1016/j.carres.2007.08.003
Luers L, Rysiewski K, Dumpitak C, Birkmann E (2012) Kinetics of advanced glycation end products formation on bovine serum albumin with various reducing sugars and dicarbonyl compounds in equimolar ratios. Rejuvenation Res 15(2):201–205. doi:10.1089/rej.2011.1284
Yoshida S, Yamada K, Hamaguchi K, Nishimura M, Hatakeyama E, Tsuchida H, Sakamoto K, Kashiwabara H, Yokoyama T, Ikeda K, Horiuchi S (1998) Immunohistochemical study of human advanced glycation end-products (AGE) and growth factors in cardiac tissues of patients on maintenance dialysis and with kidney transplantation. Clin Nephrol 49(5):273–280
Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK (2011) Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst 103(9):714–736. doi:10.1093/jnci/djr077
Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ (2010) Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 60(3):166–193. doi:10.3322/caac.20069
Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG (2005) Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol 7(2):134–153. doi:10.1215/S1152851704001115
Januel C, Fay LB, Ruggiero D, Lagarde M, Vericel E (2003) Covalent coupling of reduced glutathione with ribose: loss of cosubstrate ability to glutathione peroxidase. Biochim Biophys Acta 1620(1–3):125–132
Linetsky MD, Shipova EV, Legrand RD, Argirov OO (2005) Glucose-derived Amadori compounds of glutathione. Biochim Biophys Acta 1724(1–2):181–193. doi:10.1016/j.bbagen.2005.04.003
Krejsa CM, Franklin CC, White CC, Ledbetter JA, Schieven GL, Kavanagh TJ (2010) Rapid activation of glutamate cysteine ligase following oxidative stress. J Biol Chem 285(21):16116–16124. doi:10.1074/jbc.M110.116210
Chang LS (1996) The functional involvement of Lys-38 in the heavy subunit of rat kidney gamma-glutamylcysteine synthetase: chemical modification and mutagenesis studies. J Protein Chem 15(3):321–326
Gamcsik MP, Kasibhatla MS, Teeter SD, Colvin OM (2012) Glutathione levels in human tumors. Biomarkers 17(8):671–691. doi:10.3109/1354750X.2012.715672
Conflict of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Backos, D.S., Fritz, K.S., McArthur, D.G. et al. Glycation of Glutamate Cysteine Ligase by 2-Deoxy-d-Ribose and its Potential Impact on Chemoresistance in Glioblastoma. Neurochem Res 38, 1838–1849 (2013). https://doi.org/10.1007/s11064-013-1090-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1090-4