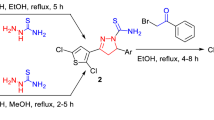
Abstract
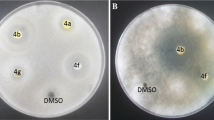
A new series of semicarbazone-triazole hybrid derivatives have been synthesized by condensation between heterocyclic aldehydes and the commercial semicarbazide hydrochloride. The in vitro antioxidant activity of these species was tested using 1,1-diphenyl-2-picrylhydrazyl radical, 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) and Ferric reducing antioxidant power assays and their antimicrobial activity against different microbial strains was carried out. Furthermore, molecular properties prediction and drug likeness were also determinated using Molinspiration. Among such derivatives, compounds (E)-2-(4-((1-(2,6-dimethylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)hydrazine carboxamide (4c), and (E)-2-(4-((1-(2-methoxyphenyl)-1-H-1,2,3-triazol-4-yl)methoxy)benzylidene)hydrazine-carboxamide (4e) exhibit excellent scavenging ability, especially with IC50 = 1.57 ± 1.66 mg/mL (4c) and IC50 = 1.82 ± 0.15 mg/mL (4e) with 1,1-diphenyl-2-picrylhydrazyl radical and IC50 = 1.90 ± 1.33 mg/mL (4c) with 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) as compared to the standards butylhydroxytoluene (IC50 = 1.60 ± 1.98 mg/mL) and Trolox (IC50 = 1.45 ± 1.33 mg/mL), respectively. The antimicrobial assay results, show that compounds 4c and 4e highlighted the most interesting profile with the potent activity was obtained against S. enteritidis (1.56-fold) and then M. luteus (1.45-fold) which are significantly higher than the positive control, chloramphenicol. By the other hand, the synthesized semicarbazone derivatives met the Lipinski’s rule criteria by presenting good drug likeness and bioactivity scores. The structure–property–activity relationships have been carried out in order to determine the effect of various substituents on the molecular and the biological properties. All these investigations confirm that our synthetic semicarbazone can be explored for generating new potential drug with good oral bioavailability.
Graphical abstract


Similar content being viewed by others
References
Ahsan MJ, Stables JP (2013) Psychomotor seizure test, neurotoxicity and in vitro neuroprotection assay of some semicarbazone analogues. Cent Nerv Syst Agents Med Chem 13:141–147
Ahsan MJ (2013) Semicarbazone analogs as anticonvulsant agents: a review. Cent Nerv Syst Agents Med Chem 13:148–158
Ahsan MJ, Khalilullah H, Yasmin S, Jadav SS, Stables JP, Govindasamy J (2013) Synthesis and anticonvulsant evaluation of 2-(substituted benzylidene/ethylidene)-N-(substituted phenyl)hydrazinecarboxamide analogues. Med Chem Res 22:2746–2754
Pandeya SN, Yogeeswari P, Stables JP (2014) Synthesis and anticonvulsant activity of 4-bromophenyl substituted aryl semicarbazones. Eur J Med Chem 35:879–886
Amir M, Ahsan MJ, Ali I (2010) Synthesis of N1-(3-chloro-4-flourophenyl)-N4-substituted semicarbazones as novel anticonvulsant agents. Indian J Chem 49B:1509–1514
Rajak H, Deshmukh R, Veerasamy R, Sharma AK, Mishra P, Kharya MD (2010) Novel semicarbazones based 2,5-disubstituted-1,3,4-oxadiazoles: one more step towards establishing four binding site pharmacophoric model hypothesis for anticonvulsant activity. Bioorg Med Chem Lett 20:4168–4172
Rajak H, Thakur BS, Singh A, Raghuvanshi K, Sah AK, Veerasamy R, Sharma PC, Pawar RS, Khary MD (2013) Novel limonene and citral based 2,5-disubstituted-1,3,4-oxadiazoles: a natural product coupled approach to semicarbazones for antiepileptic activity. Bioorg Med Chem Lett 23:864–868
Shukla S, Srivastava RS, Shrivastava SK, Sodhi A, Kumar P (2012) Synthesis, characterization and antiproliferative activity of 1,2-naphthoquinone and its derivatives. Appl Biochem Biotechnol 167:1430–1445
Liu Z, Wu S, Wang Y, Li R, Wang J, Wang L, Zhao Y, Gong P (2014) Design, synthesis and biological evaluation of novel thieno[3,2-d]pyrimidine derivatives possessing diaryl semicarbazone scaffolds as potent antitumor agents. Eur J Med Chem 87:782–793
Ahsan MJ, Amir M, Bakht MA, Samy JG, Hasan MZ, Nomani MS (2016) Synthesis and antimicrobial activity of N1-(3-chloro-4-fluorophenyl)-N4-substituted semicarbazone derivatives. Arab J Chem 9:S861–S866
Dutta S, Padhye S, Priyadarsini KI, Newton C (2005) Antioxidant and antiproliferative activity of curcumin semicarbazone. Bioorg Med Chem Lett 15:2738–2744
Sriram D, Yogeeswari P, Thirumurugan R (2004) Synthesis and anticonvulsant and neurotoxicity evaluation of N4-phthalimido phenyl (thio) semicarbazides. Bioorg Med Chem Lett 14:3923–3924
Kumar D, Beena, Khare G, Kidwai S, Tyagi AK, Singh R, Rawat DS (2014) Synthesis of novel 1,2,3-triazole derivatives of isoniazid and their in vitro and in vivo antimycobacterial activity evaluation. Eur J Med Chem 81:301–313
Kasuga NC, Sekino K, Koumo C, Shimada N, Ishikawa M (2001) Synthesis, structural characterization and antimicrobial activities of 4- and 6-coordinate nickel(II) complexes with three thiosemicarbazones and semicarbazone ligands. J Inorg Biochem 84:55–65
Afrasiabi Z, Sinn EKK, Lin W, Campana Ma Y, Padhye CS (2005) Nickel (II) complexes of naphthaquinone thiosemicarbazone and semicarbazone: synthesis, structure, spectroscopy and biological activity. J Inorg Biochem 9:1526–1531
Alomar K, Gaumet V, Allain M, Bouet G, Landreau A (2012) Synthesis, crystal structure, characterisation, and antifungal activity of 3-thiophene aldehyde semicarbazone (3STCH), 2,3-thiophene dicarboxaldehyde bis(semicarbazone) (2,3BSTCH2) and their nickel (II) complexes. J Inorg Biochem 115:36–43
Kinfe HH, Belay YH, Joseph JS, Mukwevho E (2013) Evaluation of the Influence of thiosemicarbazone–triazole hybrids on genes implicated in lipid oxidation and accumulation as potential anti-obesity agents. Bioorg Med Chem Lett 23:5275–5278
Giguère JB, Thibeault D, Cronier F, Marois JS, Auger M, Morin JF (2009) Synthesis of [2]- and [3] rotaxanes through sonogashira coupling. Tetrahedron Lett 50:5497–5500
Lal K, Yadav P, Kumar A (2016) Synthesis, characterization and antimicrobial activity of 4-((1-benzyl/phenyl-1H-1,2,3-triazol-4-yl)methoxy)benzaldehyde analogues. Med Chem Res 25:644–652
Ghannay S, Bakari S, Ghabi A, Kadri A, Msaddek M, Aouadi K (2017) Stereoselective synthesis of enantiopure N-substituted pyrrolidin-2,5-dione derivatives by 1,3-dipolar cycloaddition and assessment of their in vitro antioxidant and antibacterial activities. Bioorg Med Chem Lett 27:2302–2307
Ghannay S, Bakari S, Msaddek M, Vidal S, Kadri A, Aouadi K (2018) Design, synthesis, molecular properties and in vitro antioxidant and antibacterial potential of novel enantiopure isoxazolidine derivatives. Arab J Chem. https://doi.org/10.1016/j.arabjc.2018.03.013
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45:2615–2623
Karamać M, Koleva L, Kancheva VD, Amarowicz R (2017) The structure–antioxidant activity relationship of ferulates. Molecules 22:527–534
Khan I, Ali S, Hameed S, Rama N, Hussain M, Wadood A, Uddin R, Ul-Haq Z, Khan A (2010) Synthesis, antioxidant activities and urease inhibition of some new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Eur J Med Chem 45:5200–5207
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brahmi, J., Bakari, S., Nasri, S. et al. Synthesis and SPAR exploration of new semicarbazone-triazole hybrids in search of potent antioxidant, antibacterial and antifungal agents. Mol Biol Rep 46, 679–686 (2019). https://doi.org/10.1007/s11033-018-4523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4523-y