Abstract
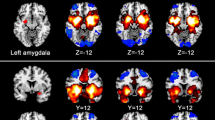
Depression and cognitive control deficits were frequently reported in concurrent end-stage renal disease (ESRD) patients. Neuroimaging studies indicated depression could be a risk factor for cognitive control deficits, and amygdala-related circuitry may play a critical role in this abnormal interaction. To investigate the potential relationship between depressive symptoms and cognitive control reduction in ESRD patients, T1-weighted and resting fMRI images were obtained in 29 ESRD patients and 29 healthy controls. Voxel-based morphometry (VBM), structural covariance (SC) analysis based on grey matter volume (GMV), and functional connectivity (FC) analysis were adopted. All subjects performed the Beck Depression Inventory (BDI) assessment and Stroop test. The patients also underwent blood biochemistry tests (urea, creatinine, phosphate, Ca2+, hematocrit, cystatin, hemoglobin). Compared with controls, GMV reductions were found mainly in the anterior cingulate cortex (ACC) and bilateral amygdala, and decreased SC was found between the amygdala and ACC in ESRD patients. This indicated that structural changes in the amygdala may be related to the GMV alterations in the ACC. Additionally, decreased FC between the amygdala and ACC was revealed in ESRD patients. Negative correlation was found between the FC of the amygdala-ACC and reaction delay during the Stroop test, but this correlation disappeared after controlling BDI. Stepwise regression analysis showed that the low level of hemoglobin was contributed to the reduced FC of the amygdala-ACC in ESRD patients. Our results demonstrated the abnormal interaction between depressive mood and cognitive control deficits in ESRD patients.


Similar content being viewed by others
References
Agganis BT, Weiner DE, Giang LM, Scott T, Tighiouart H, Griffith JL, Sarnak MJ (2010) Depression and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis 56:704–712
Angelova PR, Kasymov V, Christie I, Sheikhbahaei S, Turovsky E, Marina N, Korsak A, Zwicker J, Teschemacher AG, Ackland GL, Funk GD, Kasparov S, Abramov AY, Gourine AV (2015) Functional oxygen sensitivity of astrocytes. J Neurosci Off J Soc Neurosci 35:10460–10473
Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K (2006) Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the cardiovascular health study. Arch Gen Psychiatry 63:273–279
Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001) Conflict monitoring and cognitive control. Psychol Rev 108:624–652
Buchman AS, Tanne D, Boyle PA, Shah RC, Leurgans SE, Bennett DA (2009) Kidney function is associated with the rate of cognitive decline in the elderly. Neurology 73:920–927
Butz M, Worgotter F, van Ooyen A (2009) Activity-dependent structural plasticity. Brain Res Rev 60:287–305
Chen HJ, Zhang LJ, Lu GM (2015) Multimodality MRI findings in patients with end-stage renal disease. Biomed Res Int 2015:697402
Chen HJ, Wang YF, Qi R, Schoepf UJ, Varga-Szemes A, Ball BD, Zhang Z, Kong X, Wen J, Li X, Lu GM, Zhang LJ (2017) Altered amygdala resting-state functional connectivity in maintenance hemodialysis end-stage renal disease patients with depressive mood. Mol Neurobiol 54:2223–2233
Chou KH, Lin WC, Lee PL, Tsai NW, Huang YC, Chen HL, Cheng KY, Chen PC, Wang HC, Lin TK, Li SH, Lin WM, Lu CH, Lin CP (2015) Structural covariance networks of striatum subdivision in patients with Parkinson's disease. Hum Brain Mapp 36:1567–1584
Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol MJ, van der Wee NJ, Veltman DJ, Roelofs K (2010) Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. NeuroImage 49:963–970
Cui X, Lyness JM, Tu X, King DA, Caine ED (2007) Does depression precede or follow executive dysfunction? Outcomes in older primary care patients. Am J Psychiatry 164:1221–1228
Cukor D, Rosenthal DS, Jindal RM, Brown CD, Kimmel PL (2009) Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int 75:1223–1229
Davenport A (2008) The brain and the kidney--organ cross talk and interactions. Blood Purif 26:526–536
Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E, Habel U (2009) Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology 34:687–693
Dolcos F, McCarthy G (2006) Brain systems mediating cognitive interference by emotional distraction. J Neurosci Off J Soc Neurosci 26:2072–2079
Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashan TH, Wexler BE (2003) Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry 54:1284–1293
Doppenberg EM, Watson JC, Bullock R, Gerber MJ, Zauner A, Abraham DJ (1997) The rationale for, and effects of oxygen delivery enhancement to ischemic brain in a feline model of human stroke. Ann N Y Acad Sci 825:241–257
Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A (2007) Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130:2375–2386
Du M, Liu J, Chen Z, Huang X, Li J, Kuang W, Yang Y, Zhang W, Zhou D, Bi F, Kendrick KM, Gong Q (2014) Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci JPN 39:397–406
Ehring T, Tuschen-Caffier B, Schnulle J, Fischer S, Gross JJ (2010) Emotion regulation and vulnerability to depression: spontaneous versus instructed use of emotion suppression and reappraisal. Emotion 10:563–572
Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J (2006) Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51:871–882
Evans AC (2013) Networks of anatomical covariance. NeuroImage 80:489–504
Gallagher M, Chiba AA (1996) The amygdala and emotion. Curr Opin Neurobiol 6:221–227
Ganguli M, Du Y, Dodge HH, Ratcliff GG, Chang CC (2006) Depressive symptoms and cognitive decline in late life: a prospective epidemiological study. Arch Gen Psychiatry 63:153–160
Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG (2006) Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in chronic heart failure: outcomes and resource utilization (ANCHOR) study. Circulation 113:2713–2723
Golden CJ (1976) Identification of brain disorders by the Stroop color and word test. J Clin Psychol 32:654–658
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14:21–36
Gupta A, Lepping RJ, Yu AS, Perea RD, Honea RA, Johnson DK, Brooks WM, Burns JM (2016) Cognitive function and white matter changes associated with renal transplantation. Am J Nephrol 43:50–57
Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ (2009) Validation of depression screening scales in patients with CKD. Am J Kidney Dis 54:433–439
Hsieh TJ, Chang JM, Chuang HY, Ko CH, Hsieh ML, Liu GC, Hsu JS (2009) End-stage renal disease: in vivo diffusion-tensor imaging of silent white matter damage. Radiology 252:518–525
Krug MK, Carter CS (2010) Adding fear to conflict: a general purpose cognitive control network is modulated by trait anxiety. Cogn Affect Behav Neurosci 10:357–371
Kurella TM, Larive B, Unruh ML, Stokes JB, Nissenson A, Mehta RL, Chertow GM, Frequent Hemodialysis Network Trial G (2010) Prevalence and correlates of cognitive impairment in hemodialysis patients: the frequent hemodialysis network trials. Clin J Am Soc Nephrol : CJASN 5:1429–1438
Langevin JP, Chen JW, Koek RJ, Sultzer DL, Mandelkern MA, Schwartz HN, Krahl SE (2016) Deep brain stimulation of the basolateral amygdala: targeting technique and electrodiagnostic findings. Brain Sci 6:28–37
Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC (2006) Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. NeuroImage 31:993–1003
Li X, Cao Q, Pu F, Li D, Fan Y, An L, Wang P, Wu Z, Sun L, Li S, Wang Y (2015) Abnormalities of structural covariance networks in drug-naive boys with attention deficit hyperactivity disorder. Psychiatry Res 231:273–278
Liao W, Zhang Z, Mantini D, Xu Q, Wang Z, Chen G, Jiao Q, Zang YF, Lu G (2013) Relationship between large-scale functional and structural covariance networks in idiopathic generalized epilepsy. Brain Connect 3:240–254
Lu R, Kiernan MC, Murray A, Rosner MH, Ronco C (2015) Kidney-brain crosstalk in the acute and chronic setting. Nat Rev Nephrol 11:707–719
MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS (2000) Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288:1835–1838
Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT (1999) Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 156:675–682
Mechelli A, Friston KJ, Frackowiak RS, Price CJ (2005) Structural covariance in the human cortex. J Neurosci Off J Soc Neurosci 25:8303–8310
Modinos G, Vercammen A, Mechelli A, Knegtering H, McGuire PK, Aleman A (2009) Structural covariance in the hallucinating brain: a voxel-based morphometry study. J Psychiatry Neurosci : JPN 34:465–469
Murray AM (2009) The brain and the kidney connection: a model of accelerated vascular cognitive impairment. Neurology 73:916–917
Ochsner KN, Gross JJ (2005) The cognitive control of emotion. Trends Cogn Sci 9:242–249
Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Fishbane S, Strippoli GF (2013) Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int 84:179–191
Passamonti L, Rowe JB, Ewbank M, Hampshire A, Keane J, Calder AJ (2008) Connectivity from the ventral anterior cingulate to the amygdala is modulated by appetitive motivation in response to facial signals of aggression. NeuroImage 43:562–570
Price JL (2003) Comparative aspects of amygdala connectivity. Ann N Y Acad Sci 985:50–58
Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW (1995) Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol 355:455–469
Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH (2013) An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage 64:240–256
Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD (2009) Neurodegenerative diseases target large-scale human brain networks. Neuron 62:42–52
Sharma A, Yabes J, Al Mawed S, Wu C, Stilley C, Unruh M, Jhamb M (2016) Impact of cognitive function change on mortality in renal transplant and end-stage renal disease patients. Am J Nephrol 44:462–472
Sheikhbahaei S, Turovsky EA, Hosford PS, Hadjihambi A, Theparambil SM, Liu B, Marina N, Teschemacher AG, Kasparov S, Smith JC, Gourine AV (2018) Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nat Commun 9:370
Soriano-Mas C, Harrison BJ, Pujol J, Lopez-Sola M, Hernandez-Ribas R, Alonso P, Contreras-Rodriguez O, Gimenez M, Blanco-Hinojo L, Ortiz H, Deus J, Menchon JM, Cardoner N (2013) Structural covariance of the neostriatum with regional gray matter volumes. Brain Struct Funct 218:697–709
Subira M, Cano M, de Wit SJ, Alonso P, Cardoner N, Hoexter MQ, Kwon JS, Nakamae T, Lochner C, Sato JR, Jung WH, Narumoto J, Stein DJ, Pujol J, Mataix-Cols D, Veltman DJ, OCDBI C, Menchon JM, van den Heuvel OA, Soriano-Mas C (2016) Structural covariance of neostriatal and limbic regions in patients with obsessive-compulsive disorder. J Psychiatry Neurosci : JPN 41:115–123
Taylor PA, Gohel S, Di X, Walter M, Biswal BB (2012) Functional covariance networks: obtaining resting-state networks from intersubject variability. Brain Connect 2:203–217
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15:273–289
Wagner G, Schachtzabel C, Peikert G, Bar KJ (2015) The neural basis of the abnormal self-referential processing and its impact on cognitive control in depressed patients. Hum Brain Mapp 36:2781–2794
Wang H, Jin C, Yuan K, Shakir TM, Mao C, Niu X, Niu C, Guo L, Zhang M (2015) The alteration of gray matter volume and cognitive control in adolescents with internet gaming disorder. Front Behav Neurosci 9:64
Wojtalik JA, Eack SM, Pollock BG, Keshavan MS (2012) Prefrontal gray matter morphology mediates the association between serum anticholinergicity and cognitive functioning in early course schizophrenia. Psychiatry Res 204:61–67
Wu H, Sun H, Wang C, Yu L, Li Y, Peng H, Lu X, Hu Q, Ning Y, Jiang T, Xu J, Wang J (2017) Abnormalities in the structural covariance of emotion regulation networks in major depressive disorder. J Psychiatr Res 84:237–242
Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS (1999) Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry 56:425–430
Yan C, Zang Y (2010) DPARSF: a MATLAB toolbox for "pipeline" data analysis of resting-state fMRI. Front Syst Neurosci 4:13
Yuan K, Yu D, Bi Y, Li Y, Guan Y, Liu J, Zhang Y, Qin W, Lu X, Tian J (2016) The implication of frontostriatal circuits in young smokers: a resting-state study. Hum Brain Mapp 37:2013–2026
Zhang LJ, Wen J, Ni L, Zhong J, Liang X, Zheng G, Lu GM (2013) Predominant gray matter volume loss in patients with end-stage renal disease: a voxel-based morphometry study. Metab Brain Dis 28:647–654
Zhang R, Liu K, Yang L, Zhou T, Qian S, Li B, Peng Z, Li M, Sang S, Jiang Q, Sun G (2015) Reduced white matter integrity and cognitive deficits in maintenance hemodialysis ESRD patients: a diffusion-tensor study. Eur Radiol 25:661–668
Zheng G, Wen J, Zhang L, Zhong J, Liang X, Ke W, Kong X, Zhao T, He Y, Zuo X, Luo S, Zhang LJ, Lu GM (2014) Altered brain functional connectivity in hemodialysis patients with end-stage renal disease: a resting-state functional MR imaging study. Metab Brain Dis 29:777–786
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant Nos. 81471737, 81473603, 81571640, 81501543, 81401478, 81571751, and 81470816; and the Natural Science Foundation of Shaanxi Province of China under Grant Nos. 2017ZDJC-13.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Anmao Li, Mingxia Huang, Zengjun Zhang, Junya Mu, Jixin Liu and Ming Zhang declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical statements
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Li, A., Mu, J., Huang, M. et al. Altered amygdala-related structural covariance and resting-state functional connectivity in end-stage renal disease patients. Metab Brain Dis 33, 1471–1481 (2018). https://doi.org/10.1007/s11011-018-0254-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0254-y