Abstract
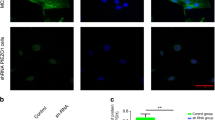
Mechano-growth factor (MGF) has emerged as an important mechanosensitive player in bone repair, but understanding of MGF function is hampered by the fact that MGF receptor and the underlying pathways remain unknown. In this study, fluorescein isothiocyanate (FITC)-labeled MGF-Ct24E (FITC-MGF) was used to determine the subcellular localization of MGF receptor in osteoblasts. After the primary osteoblasts were exposed to stretch with the strain at 10 %, and/or loaded with 50 ng/ml exogenous MGF-Ct24E, cells were incubated with the different concentrations of FITC-MGF (0.01, 0.1, and 1 mg/ml) followed by flow cytometry and laser scanning confocal microscope analysis. Our results showed that the fluorescence intensity and cell population internalizing FITC-MGF increased with the concentration of FITC-MGF. And all the cells were labeled with fluorescence at 1 mg/ml. Notably, FITC-MGF had nuclear localization when osteoblasts were exposed to stretch and/or 50 ng/ml MGF-Ct24E added, compared to the evident cytoplasmic localization in the static culture group. The nuclear localization of FITC-MGF in response to mechanical loading was found to associate with high expression of proliferating cell nuclear antigen, suggesting MGF and its receptor could serve as potential messengers that replay information in nuclei to control cell proliferation.





Similar content being viewed by others
Abbreviations
- MGF:
-
Mechano-growth factor
- IGF-I:
-
Insulin-like growth factor-I
- FITC:
-
Fluorescein isothiocyanate
- FITC-labeled MGF-Ct24E:
-
FITC-MGF
- FBS:
-
Fetal bovine serum
- HGDMEM:
-
High Glucose Dulbecco’s-Modified Eagle Medium
- PBS:
-
Phosphate-buffered saline
- BSA:
-
Bovine serum albumin
- PFA:
-
Paraformaldehyde
- PCNA:
-
Proliferating cell nuclear antigen
References
Goldspink G (2005) Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology (Bethesda) 20:232–238
Dai Z, Wu F, Yeung EW, Li Y (2010) IGF-IEc expression, regulation and biological function in different tissues. Growth Horm IGF Res 20:275–281
Philippou A, Papageorgiou E, Bogdanis G, Halapas A, Sourla A, Maridaki M, Pissimissis N, Koutsilieris M (2009) Expression of IGF-1 isoforms after exercise-induced muscle damage in humans: characterization of the MGF E peptide actions in vitro. In Vivo (Athens, Greece) 23:567–575
Hameed M, Toft AD, Pedersen BK, Harridge SD, Goldspink G (2008) Effects of eccentric cycling exercise on IGF-I splice variant expression in the muscles of young and elderly people. Scand J Med Sci Sports 18:447–452
Deng M, Zhang B, Wang K, Liu F, Xiao H, Zhao J, Liu P, Li Y, Lin F, Wang Y (2011) Mechano growth factor E peptide promotes osteoblasts proliferation and bone-defect healing in rabbits. Int Orthop 35:1099–1106
Zhang B, Xian C, Luo Y, Wang Y (2009) Expression and subcellular localization of mechano-growth factor in osteoblasts under mechanical stretch. Sci China C 52:928–934
Peng Q, Wang Y, Qiu J, Zhang B, Sun J, Lv Y, Yang L (2011) A novel mechanical loading model for studying the distributions of strain and mechano-growth factor expression. Arch Biochem Biophys 511(1–2):8–13
Zhang B, Wang Y, Yang L, Pan C, Fu Y, Deng M, Li Y (2010) The effects of MGF and E peptide on the differentiation of osteoblasts. Prog Biochem Biophys 37:304–312
Schonenberger C, Schutz A, Franco-Obregon A, Zenobi-Wong M (2011) Efficient electroporation of peptides into adherent cells: investigation of the role of mechano-growth factor in chondrocyte culture. Biotechnol Lett 33:883–888
Stavropoulou A, Halapas A, Sourla A, Philippou A, Papageorgiou E, Papalois A, Koutsilieris M (2009) IGF-1 expression in infarcted myocardium and MGF E peptide actions in rat cardiomyocytes in vitro. Mol Med 15:127–135
Yang SY, Goldspink G (2002) Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 522:156–160
Dluzniewska J, Sarnowska A, Beresewicz M, Johnson I, Srai SK, Ramesh B, Goldspink G, Gorecki DC, Zablocka B (2005) A strong neuroprotective effect of the autonomous C-terminal peptide of IGF-1 Ec (MGF) in brain ischemia. FASEB J 19:1896–1898
Mills P, Lafreniere JF, Benabdallah BF, El Fahime M, Tremblay JP (2007) A new pro-migratory activity on human myogenic precursor cells for a synthetic peptide within the E domain of the mechano growth factor. Exp Cell Res 313:527–537
Wu W, Lee WL, Wu YY, Chen D, Liu TJ, Jang A, Sharma PM, Wang PH (2000) Expression of constitutively active phosphatidylinositol 3-kinase inhibits activation of caspase 3 and apoptosis of cardiac muscle cells. J Biol Chem 275:40113–40119
Bertrand FE, Steelman LS, Chappell WH, Abrams SL, Shelton JG, White ER, Ludwig DL, McCubrey JA (2006) Synergy between an IGF-1R antibody and Raf/MEK/ERK and PI3 K/Akt/mTOR pathway inhibitors in suppressing IGF-1R-mediated growth in hematopoietic cells. Leukemia 20:1254–1260
Matheny RW Jr, Nindl BC, Adamo ML (2010) Minireview: mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology 151:865–875
Chew SL, Lavender P, Clark AJ, Ross RJ (1995) An alternatively spliced human insulin-like growth factor-I transcript with hepatic tissue expression that diverts away from the mitogenic IBE1 peptide. Endocrinology 136:1939–1944
Robey PG, Termine JD (1985) Human bone cells in vitro. Calcif Tissue Int 37:453–460
Li A, Kaur P (2005) FACS enrichment of human keratinocyte stem cells. Methods Mol Biol 289:87–96
Naryzhny SN (2008) Proliferating cell nuclear antigen: a proteomics view. Cell Mol Life Sci 65:3789–3808
Pederson T (1998) Growth factors in the nucleolus? J Cell Biol 143:279–281
Lee CY, Ryan RJ (1972) Luteinizing hormone receptors: specific binding of human luteinizing hormone to homogenates of luteinized rat ovaries. Proc Natl Acad Sci USA 69:3520–3523
Bradley ME, Bond ME, Manini J, Brown Z, Charlton SJ (2009) SB265610 is an allosteric, inverse agonist at the human CXCR2 receptor. Br J Pharmacol 158:328–338
Hill M, Wernig A, Goldspink G (2003) Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat 203:89–99
Kandalla PK, Goldspink G, Butler-Browne G, Mouly V (2011) Mechano Growth Factor E peptide (MGF-E), derived from an isoform of IGF-1, activates human muscle progenitor cells and induces an increase in their fusion potential at different ages. Mech Ageing Dev 132:154–162
Hill M, Goldspink G (2003) Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549:409–418
Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, Protheroe AS, Howarth M, Macaulay VM (2011) Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res 70:6412–6419
Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC (2001) Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol 3:802–808
Lo HW, Ali-Seyed M, Wu Y, Bartholomeusz G, Hsu SC, Hung MC (2006) Nuclear-cytoplasmic transport of EGFR involves receptor endocytosis, importin beta1 and CRM1. J Cell Biochem 98:1570–1583
Bodzin AS, Wei Z, Hurtt R, Gu T, Doria C (2012) Gefitinib resistance in HCC Mahlavu cells: upregulation of CD133 expression, activation of IGF-1R signaling pathway, and enhancement of IGF-1R nuclear translocation. J Cell Physiol 227(7):2947–2952
Baserga R, Peruzzi F, Reiss K (2003) The IGF-1 receptor in cancer biology. Int J Cancer 107:873–877
Hartog H, Wesseling J, Boezen HM, van der Graaf WT (2007) The insulin-like growth factor 1 receptor in cancer: old focus, new future. Eur J Cancer 43:1895–1904
Santos A, Bakker AD, Willems HM, Bravenboer N, Bronckers AL, Klein-Nulend J (2011) Mechanical loading stimulates BMP7, but not BMP2, production by osteocytes. Calcif Tissue Int 89:318–326
Tang L, Lin Z, Li YM (2006) Effects of different magnitudes of mechanical strain on Osteoblasts in vitro. Biochem Biophys Res Commun 344:122–128
Myers KA, Rattner JB, Shrive NG, Hart DA (2007) Osteoblast-like cells and fluid flow: cytoskeleton-dependent shear sensitivity. Biochem Biophys Res Commun 364:214–219
Haudenschild DR, Chen J, Pang N, Lotz MK, D’Lima DD (2011) Rho kinase-dependent activation of SOX9 in chondrocytes. Arthritis Rheum 62:191–200
Li P Jr, Ma YC Jr, Shen HL Sr, Han H Jr, Wang J Sr, Cheng HJ Jr, Wang CF Sr, Xia YY Sr (2011) Cytoskeletal reorganization mediates fluid shear stress-induced ERK5 activation in osteoblastic cells. Cell Biol Int 36(3):229–236
Whitehead J, Vignjevic D, Futterer C, Beaurepaire E, Robine S, Farge E (2008) Mechanical factors activate beta-catenin-dependent oncogene expression in APC mouse colon. HFSP J 2:286–294
Kanazawa T, Furumatsu T, Hachioji M, Oohashi T, Ninomiya Y, Ozaki T (2012) Mechanical stretch enhances COL2A1 expression on chromatin by inducing SOX9 nuclear translocalization in inner meniscus cells. J Orthop Res 30(3):468–474
Takeda K, Tonthat NK, Glover T, Xu W, Koonin EV, Yanagida M, Schumacher MA (2011) Implications for proteasome nuclear localization revealed by the structure of the nuclear proteasome tether protein Cut8. Proc Natl Acad Sci USA 108:16950–16955
Dupin I, Etienne-Manneville S (2011) Nuclear positioning: mechanisms and functions. Int J Biochem Cell Biol 43(12):1698–1707
Nalaskowski MM, Metzner A, Brehm MA, Labiadh S, Brauer H, Grabinski N, Mayr GW, Jucker M (2012) The inositol 5-phosphatase SHIP1 is a nucleo-cytoplasmic shuttling protein and enzymatically active in cell nuclei. Cell Signal 24(3):621–628
Zhao SL, Hong J, Xie ZQ, Tang JT, Su WY, Du W, Chen YX, Lu R, Sun DF, Fang JY (2011) TRAPPC4-ERK2 interaction activates ERK1/2, modulates its nuclear localization and regulates proliferation and apoptosis of colorectal cancer cells. PLoS One 6:e23262
Provenzano MJ, Minner SA, Zander K, Clark JJ, Kane CJ, Green SH, Hansen MR (2011) p75(NTR) expression and nuclear localization of p75(NTR) intracellular domain in spiral ganglion Schwann cells following deafness correlate with cell proliferation. Mol Cell Neurosci 47:306–315
Acknowledgments
This work was supported by grant from the National Natural Science Foundation of China (11032012, 30870609), Natural Science Foundation of CQ CSTC (2009BB4025), Science and Technology Program of CQ CSTC (2009AB5174) and Foundation of Chongqing Municipal Education Commission (KJ091415).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, Q., Qiu, J., Sun, J. et al. The nuclear localization of MGF receptor in osteoblasts under mechanical stimulation. Mol Cell Biochem 369, 147–156 (2012). https://doi.org/10.1007/s11010-012-1377-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1377-9