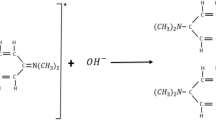
Abstract
The rate constant of malachite green (MG+) alkaline fading was measured in water–ethanol–ethylene glycol ternary mixtures. This reaction was studied under pseudo-first-order conditions at 283–303 K. In each series of experiments, the concentration of ethanol was kept constant and the concentration of ethylene glycol was changed. It was shown that due to hydrogen bonding and hydrophobic interaction between MG+ and alcohol molecules the observed reaction rate constant, \( k_{\text{obs}} \), increased in the water–ethanol–ethylene glycol ternary mixtures. The fundamental rate constants of MG+ fading in these solutions (\( k_{1} \), \( k_{ - 1} \) and \( k_{2} \)) were obtained by the SESMORTAC model. Analysis of \( k_{1} \) and \( k_{2} \) values in solutions containing constant ethanol concentrations show that in low concentrations of ethylene glycol, hydrogen bonding formed between ethanol and ethylene glycol molecules and in high concentrations of ethylene glycol, ethanol as a solvent for ethylene glycol affected the reaction rate.







Similar content being viewed by others
Abbreviations
- SESMORTAC:
-
Study of effect of solvent mixture on the one-step reaction rates using the transition state theory and cage effect
- ACSM:
-
Activated complex formed in the second mechanism
- ACSMbm :
-
ACSM formed at the bm point
- ACSM1 :
-
ACSM formed in the first zone
- AC:
-
Activated complex
- n :
-
Number of molecules of solvent 1 in the solvent cage of transition state that are replaced by the same number of solvent 2 molecules in the transition state
- mc :
-
mechanism change
- \( k_{mc(i - 1)} \) :
-
Rate constant at the \( mc_{i - 1} \) point
- \( k_{bm} \) :
-
Observed rate constant
- \( v \) :
-
Reaction rate
- bm :
-
Binary mixture
- ter :
-
Ternary mixture
- i :
-
The ith zone
References
Abraham, M.H.: Solvent effects on reaction rates. Pure Appl. Chem. 57, 1055–1064 (1985)
Um, I.H., Park, Y.M., Shin, E.H.: The effect of solvent on reaction rates and equilibria for the reactions of p-nitrophenyl acetate with alicyclic secondary amines in H2O and DMSO. Bull. Korean Chem. Soc. 20, 392–394 (1999)
Um, I.H., Shin, E.H., Kwon, D.S.: The effect of solvent on reactions of p-nitrophenyl acetate with alicyclic secondary amines and with anionic nucleophiles in MeCN–H2O mixtures of varying compositions. Bull. Korean Chem. Soc. 17, 234–239 (1996)
Mora-Diez, N., Keller, S., Alvarez-Idaboy, J.R.: The Baeyer–Villiger reaction: Solvent effects on reaction mechanisms. Org. Biomol. Chem. 7, 3682–3690 (2009)
Hazarika, S., Goswami, P., Dutta, N.N.: Lipase catalysed ttransesterification of 2-o-benzylglycerol with vinyl acetate: Solvent effect. Chem. Eng. J. 94, 1–10 (2003)
Gregory, P.: Dye and dye intermediates. In: Kroschwitz, J.I. (ed.) Encyclopedia of Chemical Technology, vol. 8, p. 544. Wiley, Indianapolis (1993)
Duxbury, D.F.: The photochemistry and photophysics of triphenylmethane dyes in solid and liquid media. Chem. Rev. 93, 381–433 (1993)
http://en.wikipedia.org/wiki/malachite_green (23 October 2012)
Samiey, B., Alizadeh, K., Mousavi, M.F., Alizadeh, N.: Study of kinetics of bromophenol blue fading in alcohol–water binary mixtures by SESMORTAC model. Bull. Korean Chem. Soc. 26, 384–392 (2005)
Samiey, B., Raoof Toosi, A.: Kinetics of malachite green fading in alcohol–water binary mixtures. Int. J. Chem. Kinet. 42, 508–518 (2010)
Ritchie, C.D.: Cation–anion combination reactions. XIII.c of the reactions of nucleophiles with esters. J. Am. Chem. Soc. 97, 1170–1179 (1975)
Ritchie, C.D., Wright, D.J., Huang, D.S., Kamego, A.A.: Cation–anion combination reaction. XII. Rates, equilibriums, and activation parameters for reactions of triarylmethyl cations in aqueous solution. J. Am. Chem. Soc. 97, 1163–1170 (1975)
Caetano, W., Tabak, M.: Interaction of chlorpromazine and trifluoperazine with anionic sodium dodecyl sulfate (SDS) micelles: Electronic absorption and fluorescence studies. J. Colloid Interface Sci. 225, 69–81 (2000)
Hughes, E.D.: Mechanism and kinetics of substitution at a saturated carbon atom. Trans. Faraday Soc. 37, 603–631 (1941)
Ingold, C.K.: Structure and Mechanism in Organic Chemistry. Bell, London (1993)
Burton, C.A.: Nucleophilic Substitution at a Saturated Carbon Atom. Elsevier, London (1963)
Wyman, J.: Dielectric constants: ethanol–diethyl ether and urea–water solutions between 0 and 50. J. Am. Chem. Soc. 55, 4116–4121 (1933)
Bhattacharya, A., Das, A.K., Kundu, K.K.: Determination of absolute transfer free energies of hydroxyl ion from water to aqueous 2-methoxy ethanol. Can. J. Chem. 59, 1153–1159 (1981)
Datta, J., Kundu, K.K.: Thermodynamics of autoionization of aqueous tetrahydrofuran and 1,2-dimethoxyethane and the structuredness of solvents. Can. J. Chem. 59, 3141–3148 (1981)
Marcus, Y.: The Properties of Solvents. Wiley (1999)
Rauf, M.A., Soliman, A.A., Khattab, M.: Solvent effect on the spectral properties of neutral red. Chem. Cent. J. 2, 19–27 (2008)
Fita, P., Fedoseeva, M., Vauthey, E.: Ultrafast excited-state dynamics of eosin B: A potential probe of the hydrogen-bonding properties of the environment. J. Phys. Chem. A 115, 2465–2470 (2011)
Reichardt, C.: Solvents and Solvent Effects in Organic Chemistry. 2nd edn., pp. 189–207. VCH Publishers (1998)
Zaghloul, A.A.: Solvent effects on the kinetics of aquation of κ-chloro(diethylenetriamine) (ethylenediamine)cobalt(III) ion in mixed aqueous solvents. Int. J. Chem. Kinet. 29, 431–436 (1997)
Ismail, A.M., Zaghloul, A.A.: Kinetics and mechanism of isatin ring opening in aqueous binary mixtures of methanol and acetonitrile cosolvents. Int. J. Chem. Kinet. 30, 463–469 (1998)
El-Subruiti, G.M.: Medium effects on the initial and the transition states upon the solvolysis of trans-dichlorobis(N-methyl-ethylenediamine)cobalt(III) complex in water–mixed solvents. Int. J. Chem. Kinet. 34, 495–499 (2002)
El-Subruiti, G.M., Chehata, A.K., Massoud, S.S.: Influence of the solvent structure on the aquation of κ-chloro(diethylenetriamine)(ethylenediamine) cobalt(III) ion in water and mixed aqueous solvents. Int. J. Chem. Kinet. 34, 1–6 (2002)
Laidler, K.J., Landskroener, P.A.: The influence of the solvent on reaction rates. Trans. Faraday Soc. 52, 200–210 (1956)
Åkerlof, G.: Dielectric constants of some organic solvent–water mixtures at various temperatures. J. Am. Chem. Soc. 54, 4125–4139 (1932)
CRC Handbook of Chemistry and Physics. In: Lide, D.L. (ed), 87th edn., Section 6, pp. 175–176. CRC Press/Taylor and Francis, Boca Raton, FL (2006)
Matulis, D.: Thermodynamics of the hydrophobic effect. III. Condensation and aggregation of alkanes, alcohols, and alkylamines. Biophys. Chem. 93, 67–82 (2001)
Reichardt, C.: Solvents and Solvent Effects in Organic Chemistry. 2nd edn., pp. 25–26. VCH Publishers (1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samiey, B., Ahmadi, S. Study of Malachite Green Fading in Water–Ethanol–Ethylene Glycol Ternary Mixtures. J Solution Chem 42, 151–164 (2013). https://doi.org/10.1007/s10953-012-9916-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9916-2