Abstract
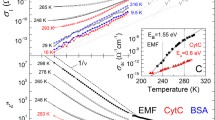
Employing optical spectroscopy we have performed a comparative study of the dielectric response of extracellular matrix and filaments of electrogenic bacteria Shewanella oneidensis MR-1, cytochrome c, and bovine serum albumin. Combining infrared transmission measurements on thin layers with data of the terahertz spectra, we obtain the dielectric permittivity and AC conductivity spectra of the materials in a broad frequency band from a few cm−1 up to 7000 cm−1 in the temperature range from 5 to 300 K. Strong absorption bands are observed in the three materials that cover the range from 10 to 300 cm−1 and mainly determine the terahertz absorption. When cooled down to liquid helium temperatures, the bands in Shewanella oneidensis MR-1 and cytochrome c reveal a distinct fine structure. In all three materials, we identify the presence of liquid bound water in the form of librational and translational absorption bands at ≈ 200 and ≈ 600 cm−1, respectively. The sharp excitations seen above 1000 cm−1 are assigned to intramolecular vibrations.






Similar content being viewed by others
Notes
The formal amount of water in EMF is higher that the one in CytC and BSA. However, the water in our samples is in three different states: bulk, loosely bound and bound. We assume, that phenomena described here are related to the behavior of loosely bound water, the amount of which is lower in EMF. For more details, see [20]
References
Veazey, J.P., Reguera, G., Tessmer, S.H.: Electronic properties of conductive pili of the metal-reducing bacterium Geobacter sulfurreducens probed by scanning tunneling microscopy. Phys. Rev. E 84, 1–4 (2011). https://doi.org/10.1103/PhysRevE.84.060901
Leung, K.M., Wanger, G., El-Naggar, M.Y., Gorby, Y., Southam, G., Lau, W.M., Yang, J.: Shewanella oneidensis MR-1 bacterial nanowires exhibit p-type, tunable electronic behavior. Nano Lett. 13, 2407–2411 (2013). https://doi.org/10.1021/nl400237p
El-Naggar, M.Y., Wanger, G., Leung, K.M., Yuzvinsky, T.D., Southam, G., Yang, J., Lau, W.M., Nealson, K.H., Gorby, Y.A.: Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc. Natl. Acad. Sci. U. S. A. 107, 18127–18131 (2010). https://doi.org/10.1073/pnas.1004880107
Malvankar, N.S., Vargas, M., Nevin, K.P., Franks, A.E., Leang, C., Kim, B.-C., Inoue, K., Mester, T., Covalla, S.F., Johnson, J.P., Rotello, V.M., Tuominen, M.T., Lovley, D.R.: Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 6, 573–579 (2011). https://doi.org/10.1038/nnano.2011.119
Malvankar, N.S., Yalcin, S.E., Tuominen, M.T., Lovley, D.R.: Visualization of charge propagation along individual pili proteins using ambient electrostatic force microscopy. Nat. Nanotechnol. 9, 1012–1017 (2014). https://doi.org/10.1038/nnano.2014.236
Pirbadian, S., Barchinger, S.E., Leung, K.M., Byun, H.S., Jangir, Y., Bouhenni, R.A., Reed, S.B., Romine, M.F., Saffarini, D.A., Shi, L., Gorby, Y.A., Golbeck, J.H., El-Naggar, M.Y.: Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc. Natl. Acad. Sci. U. S. A. 111, 12883–12888 (2014). https://doi.org/10.1073/pnas.1410551111
Subramanian, P., Pirbadian, S., El-Naggar, M.Y., Jensen, G.J.: The ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryo-tomography. bioRxiv. (2017)
Malvankar, N.S., Vargas, M., Nevin, K., Tremblay, P., Evans-Lutterodt, K., Nykypanchuk, D.: Structural basis for metallic-like conductivity in microbial nanowires. mBio 6(2), e00084 (2015). https://doi.org/10.1128/mBio.00084-15
Xiao, K., Malvankar, N.S., Shu, C., Martz, E., Lovley, D.R., Sun, X.: Low-energy atomic models suggesting a pilus structure that could account for electrical conductivity of Geobacter sulfurreducens pili. Sci. Rep. 6, 23385 (2016). https://doi.org/10.1038/srep23385
Malvankar, N.S., Lovley, D.R.: Microbial nanowires: a new paradigm for biological electron transfer and bioelectronics. ChemSusChem 5, 1039–1046 (2012). https://doi.org/10.1002/cssc.201100733
Adhikari, R.Y., Malvankar, N.S., Tuominen, M.T., Lovley, D.R.: Conductivity of individual Geobacter pili. RSC Adv. 6, 8354–8357 (2016). https://doi.org/10.1039/C5RA28092C
Vargas, M., Malvankar, N.S., Tremblay, P.-L., Leang, C., Smith, J.A., Patel, P., Snoeyenbos-West, O., Synoeyenbos-West, O., Nevin, K.P., Lovley, D.R.: Aromatic amino acids required for pili conductivity and long-range extracellular electron transport in Geobacter sulfurreducens. mBio 4, e00105 (2013). https://doi.org/10.1128/mBio.00105-13
Smith, D.M.A., Rosso, K.M.: Possible dynamically gated conductance along heme wires in bacterial multiheme cytochromes. J. Phys. Chem. B 118, 8505–8512 (2014). https://doi.org/10.1021/jp502803y
El-Naggar, M.Y., Gorby, Y.A., Xia, W., Nealson, K.H.: The molecular density of states in bacterial nanowires. Biophys. J. 95, L10–L12 (2008). https://doi.org/10.1529/biophysj.108.134411
Breuer, M., Rosso, K.M., Blumberger, J., Butt, J.N.: Multi-haem cytochromes in Shewanella oneidensis MR-1: structures, functions and opportunities. J. R. Soc. Interface 12, 20141117 (2015). https://doi.org/10.1098/rsif.2014.1117
Yan, H., Chuang, C., Zhugayevych, A., Tretiak, S., Dahlquist, F.W., Bazan, G.C.: Inter-aromatic distances in Geobacter sulfurreducens pili relevant to biofilm charge transport. Adv. Mater. 27, 1908–1911 (2015). https://doi.org/10.1002/adma.201404167
Grebenko, A., Dremov, V., Barzilovich, P., Bubis, A., Sidoruk, K., Voeikova, T., Gagkaeva, Z., Chernov, T., Korostylev, E., Gorshunov, B., Motovilov, K.: Impedance spectroscopy of single bacterial nanofilament reveals water-mediated charge transfer. PLoS ONE 13, 1–17 (2018). https://doi.org/10.1371/journal.pone.0191289
Zaytsev, K.I., Kudrin, K.G., Karasik, V.E., Reshetov, I. V., Yurchenko, S.O.: In vivo terahertz spectroscopy of pigmentary skin nevi: pilot study of non-invasive early diagnosis of dysplasia. Appl. Phys. Lett. 106, (2015). https://doi.org/10.1063/1.4907350
Dressel, M., Gruner, G.: Electrodynamics of Solids. Cambridge University Press, Cambridge (2002)
Motovilov, K.A., Savinov, M., Zhukova, E.S., Pronin, A.A., Gagkaeva, Z. V., Grinenko, V., Sidoruk, K. V., Voeikova, T.A., Barzilovich, P.Y., Grebenko, A.K., Lisovskii, S. V., Torgashev, V.I., Bednyakov, P., Pokorný, J., Dressel, M., Gorshunov, B.P.: Observation of dielectric universalities in albumin, cytochrome c and Shewanella oneidensis MR-1 extracellular matrix. Sci. Rep. 7, 15731 (2017). https://doi.org/10.1038/s41598-017-15693-y
Logan, B.E., Hamelers, B., Rozendal, R., Schröder, U., Keller, J., Freguia, S., Aelterman, P., Verstraete, W., Rabaey, K.: Microbial fuel cells: methodology and technology. Environ. Sci. Technol. 40, 5181–5192 (2006)
Richardson, D.J., Butt, J.N., Fredrickson, J.K., Zachara, J.M., Shi, L., Edwards, M.J., White, G., Baiden, N., Gates, A.J., Marritt, S.J., Clarke, T.A.: The “porin-cytochrome” model for microbe-to-mineral electron transfer. Mol. Microbiol. 85, 201–212 (2012). https://doi.org/10.1111/j.1365-2958.2012.08088.x
Gorshunov, B., Volkov, A., Spektor, I., Prokhorov, A., Mukhin, A., Dressel, M., Uchida, S., Loidl, A.: Terahertz BWO-spectrosopy. Int. J. Infrared Millimeter Waves 26, 1217–1240 (2005). https://doi.org/10.1007/s10762-005-7600-y
Born, M., Wolf, E.: Principles of Optics. Pergamon, Oxford (1980)
Downing, H.D., Williams, D.: Optical constants of water in the infrared. J. Geophys. Res. 80, 1656–1661 (1975). https://doi.org/10.1029/JC080i012p01656
Zelsmann, H.R.: Temperature dependence of the optical constants for liquid H2O and D2O in the far IR region. J. Mol. Struct. 350, 95–114 (1995). https://doi.org/10.1016/0022-2860(94)08471-S
Liebe, H.J., Hufford, G.A., Manabe, T.: A model for the complex permittivity of water at frequencies below 1 THz. Int. J. Infrared Millimeter Waves 12, 659–675 (1991). https://doi.org/10.1007/BF01008897
Grdadolnik, J., Marechal, Y.: Bovine serum albumin observed by infrared spectrometry. II. Hydration mechanisms and interaction configurations of embedded H2O molecules. Biopolymers 62, 54–67 (2001). https://doi.org/10.1002/1097-0282(2001)62:1<54::AID-BIP70>3.0.CO;2-4
Grdadolnik, J., Marechal, Y.: Bovine serum albumin observed by infrared spectrometry. I. Methodology, structural investigation, and water uptake. Biopolymers 62, 40–53 (2001). https://doi.org/10.1002/1097-0282(2001)62:1<40::AID-BIP60>3.0.CO;2-C
Thielges, M.C., Zimmermann, J., Dawson, P.E., Romesberg, F.E.: The determinants of stability and folding in evolutionarily diverged cytochromes c. J. Mol. Biol. 388, 159–167 (2009). https://doi.org/10.1016/j.jmb.2009.02.059
Heimburg, T., Marsh, D.: Investigation of secondary and tertiary structural changes of cytochrome c in complexes with anionic lipids using amide hydrogen exchange measurements: an FTIR study. Biophys. J. 65, 2408–2417 (1993). https://doi.org/10.1016/S0006-3495(93)81299-2
Ye, M., Zhang, Q.-L., Li, H., Weng, Y.-X., Wang, W.-C., Qiu, X.-G.: Infrared spectroscopic discrimination between the loop and α-helices and determination of the loop diffusion kinetics by temperature-jump time-resolved infrared spectroscopy for cytochrome c. Biophys. J. 93, 2756–2766 (2007). https://doi.org/10.1529/biophysj.107.106799
von Hippel, A.R.: The dielectric relaxation spectra of water, ice, and aqueous solutions, and their interpretation. I. Critical survey of the status-quo for water. IEEE Trans. Electr. Insul. 23, 801–816 (1988). https://doi.org/10.1109/14.8745
Eisenberg, D., Kautzmann, W.: The Structure and Properties of Water. Oxford University Press, New York (1969)
Yamamoto, N., Ohta, K., Tamura, A., Tominaga, K.: Broadband dielectric spectroscopy on lysozyme in the sub-gigahertz to terahertz frequency regions: effects of hydration and thermal excitation. J. Phys. Chem. B 120, 4743–4755 (2016). https://doi.org/10.1021/acs.jpcb.6b01491
Urabe, H., Sugawara, Y., Ataka, M., Rupprecht, A.: Low-frequency Raman spectra of lysozyme crystals and oriented DNA films: dynamics of crystal water. Biophys. J. 74, 1533–1540 (1998). https://doi.org/10.1016/S0006-3495(98)77865-8
Nakanishi, M., Sokolov, A.P.: Protein dynamics in a broad frequency range: dielectric spectroscopy studies. J. Non-Cryst. Solids 407, 478–485 (2015). https://doi.org/10.1016/j.jnoncrysol.2014.08.057
Khodadadi, S., Pawlus, S., Sokolov, A.P.: Influence of hydration on protein dynamics: combining dielectric and neutron scattering spectroscopy data. J. Phys. Chem. B 112, 14273–14280 (2008). https://doi.org/10.1021/jp8059807
Bertie, J.E., Labbé, H.J., Whalley, E.: Absorptivity of ice I in the range 4000–30 cm−1. J. Chem. Phys. 50, 4501–4520 (1969). https://doi.org/10.1063/1.1670922
Buckingham, A.D.: The hydrogen bond, and the structure and properties of H20 and (H20)2. J. Mol. Struct. 250, 111–118 (1991). https://doi.org/10.1016/0022-2860(91)85023-V
Bagchi, B.: Water dynamics in the hydration layer around proteins and micelles. (2005). https://doi.org/10.1021/CR020661+
Qvist, J., Persson, E., Mattea, C., Halle, B.: Time scales of water dynamics at biological interfaces: peptides, proteins and cells. Faraday Discuss. 141, 131–144. discussion 175-207 (2009)
Sasisanker, P., Weingärtner, H.: Hydration dynamics of water near an amphiphilic model peptide at low hydration levels: a dielectric relaxation study. ChemPhysChem 9, 2802–2808 (2008). https://doi.org/10.1002/cphc.200800508
Khodadadi, S., Sokolov, A.P.: Protein dynamics: from rattling in a cage to structural relaxation. Soft Matter 11, 4984–4998 (2015). https://doi.org/10.1039/C5SM00636H
He, Y., Chen, J.-Y., Knab, J.R., Zheng, W., Markelz, A.G.: Evidence of protein collective motions on the picosecond timescale. Biophys. J. 100, 1058–1065 (2011). https://doi.org/10.1016/j.bpj.2010.12.3731
Markelz, A.G., Knab, J.R., Chen, J.Y., He, Y.: Protein dynamical transition in terahertz dielectric response. Chem. Phys. Lett. 442, 413–417 (2007). https://doi.org/10.1016/j.cplett.2007.05.080
Chen, J.-Y., Knab, J.R., Cerne, J., Markelz, A.G.: Large oxidation dependence observed in terahertz dielectric response for cytochrome c. Phys. Rev. E 72, 40901 (2005). https://doi.org/10.1103/PhysRevE.72.040901
Yamamoto, K., Tominaga, K., Sasakawa, H., Tamura, A., Murakami, H., Ohtake, H., Sarukura, N.: Far-infrared absorption measurements of polypeptides and cytochrome c by THz radiation. Bull. Chem. Soc. Jpn. 75, 1083–1092 (2002). https://doi.org/10.1246/bcsj.75.1083
Markelz, A., Roitberg, A., Heilweil, E.: Pulsed terahertz spectroscopy of DNA, bovine serum albumin and collagen between 0.1 and 2.0 THz. Chem. Phys. Lett. 320, 42–48 (2000). https://doi.org/10.1016/S0009-2614(00)00227-X
Mernea, M., Calborean, O., Grigore, O., Dascalu, T., Mihailescu, D.F.: Validation of protein structural models using THz spectroscopy: a promising approach to solve three-dimensional structures. Opt. Quant. Electron. 46, 505–514 (2014). https://doi.org/10.1007/s11082-013-9872-0
Acbas, G., Niessen, K.A., Snell, E.H., Markelz, A.G.: Optical measurements of long-range protein vibrations. Nat. Commun. 6, 3076 (2014). https://doi.org/10.1038/ncomms4076
Balu, R., Zhang, H., Zukowski, E., Chen, J.-Y., Markelz, A.G., Gregurick, S.K.: Terahertz spectroscopy of bacteriorhodopsin and rhodopsin: similarities and differences. Biophys. J. 94, 3217–3226 (2008). https://doi.org/10.1529/biophysj.107.105163
Xu, J., Plaxco, K.W., Allen, S.J.: Probing the collective vibrational dynamics of a protein in liquid water by terahertz absorption spectroscopy. Protein Sci. 15, 1175–1181 (2006). https://doi.org/10.1110/ps.062073506
Markelz, A., Whitmire, S., Hillebrecht, J., Birge, R.: THz time domain spectroscopy of biomolecular conformational modes. Phys. Med. Biol. 47, 3797–3805 (2002). https://doi.org/10.1088/0031-9155/47/21/318
Balog, E., Becker, T., Oettl, M., Lechner, R., Daniel, R., Finney, J., Smith, J.C.: Direct determination of vibrational density of states change on ligand binding to a protein. Phys. Rev. Lett. 93, 28103 (2004). https://doi.org/10.1103/PhysRevLett.93.028103
MacKerell, A.D., Bashford, D., Bellott, M., Dunbrack, R.L., Evanseck, J.D., Field, M.J., Fischer, S., Gao, J., Guo, H., Ha, S., Joseph-McCarthy, D., Kuchnir, L., Kuczera, K., Lau, F.T., Mattos, C., Michnick, S., Ngo, T., Nguyen, D.T., Prodhom, B., Reiher, W.E., Roux, B., Schlenkrich, M., Smith, J.C., Stote, R., Straub, J., Watanabe, M., Wiórkiewicz-Kuczera, J., Yin, D., Karplus, M.: All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998). https://doi.org/10.1021/jp973084f
Smith, J.C.: Protein dynamics: comparison of simulations with inelastic neutron scattering experiments. Q. Rev. Biophys. 24, 227–291 (1991)
Hayward, S., Kitao, A., Hirata, F., Gō, N.: Effect of solvent on collective motions in globular protein. J. Mol. Biol. 234, 1207–1217 (1993). https://doi.org/10.1006/jmbi.1993.1671
Go, N., Noguti, T., Nishikawa, T.: Dynamics of a small globular protein in terms of low-frequency vibrational modes. Proc. Natl. Acad. Sci. U. S. A. 80, 3696–3700 (1983)
Rasmussen, B.F., Stock, A.M., Ringe, D., Petsko, G.A.: Crystalline ribonuclease a loses function below the dynamical transition at 220 K. Nature 357, 423–424 (1992). https://doi.org/10.1038/357423a0
Sokolov, A.P., Roh, J.H., Mamontov, E., García Sakai, V.: Role of hydration water in dynamics of biological macromolecules. Chem. Phys. 345, 212–218 (2008). https://doi.org/10.1016/j.chemphys.2007.07.013
He, Y., Ku, P., Knab, J., Chen, J., Markelz, A.: Protein dynamical transition does not require protein structure. Phys. Rev. Lett. 101, 178103 (2008). https://doi.org/10.1103/PhysRevLett.101.178103
Acknowledgements
This work was supported by the Ministry of Education and Science of the Russian Federation (Projects N3.9896.2017/BY, 5-100) and by MIPT visiting professors grant. The authors acknowledge K.A. Motovilov for fruitful discussions. We acknowledge discussions with V.I. Borshchevskiy, V.I. Gordelii, Yu. Feldman, V.V. Lebedev, S. Tretiak, G.A. Tsirlina, A. Zhugayevych.
Author information
Authors and Affiliations
Contributions
T.A.V. and B.P.G. designed the research; K.V.S. and T.A.V. performed cultivation of Shewanella oneidensis MR-1, A.K.G. prepared EMF, Z.V.G., E.S.Z, V.G., K.V.S. and A.K.G. performed research; Z.V.G., E.S.Z. and V.G. analyzed data; B.P.G and M.D. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Gagkaeva, Z.V., Zhukova, E.S., Grinenko, V. et al. Terahertz-infrared spectroscopy of Shewanella oneidensis MR-1 extracellular matrix. J Biol Phys 44, 401–417 (2018). https://doi.org/10.1007/s10867-018-9497-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-018-9497-4