Abstract
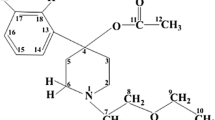
The base form of the local anaesthetic kazcaine (BFK, [1-(2-ethoxyethyl)-4-ethynyl-4-benzoyloxypiperidine, C18H23NO3]) and β-cyclodextrin (β-CD) co-crystallized as BFK:β-CD inclusion complex in 1:2 M ratio from a mixture of water and ethanol while the filtered mother liquor yielded crystals of free BFK. X-ray diffraction showed that the crystals of BFK and its inclusion complex with β-CD belong to monoclinic (P21/c) and triclinic (P1) space groups, respectively. The crystals of free BFK are stabilized by pairs of C–H⋯O, C–H⋯π and ≡C–H⋯O type interactions and van der Waals contacts. In the 1:2 BFK:β-CD complex the two β-CD molecules are in hydrogen-bonding contact with their primary hydroxyl groups, the 1-(2-ethoxyethyl)-4-ethynyl-piperidine moiety being located in one and the benzoyloxy group of BFK in the other β-CD. This crystal structure is of the channel-type, the β-CD molecules of the 1:2 BFK:β-CD complex interacting with their secondary hydroxyl groups. The pharmacological activities of the 1:2 BFK/β-CD inclusion complex have been determined in mice, rats, porpoises and rabbits and compare favourably with those of kazcaine, procaine, dicaine, lidocaine and trimecaine. The methods used include terminal (superficial), infiltration, conduction anaesthesia, and acute toxicity.




Similar content being viewed by others
References
Saenger, W.: Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. Engl. 19, 344–362 (1980)
Dodziuk, H.: Cyclodextrins and Their Complexes. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim (2006)
Lindner, K., Saenger, W.: β-Cyclodextrin dodecahydrate: crowding of water molecules within a hydrophobic cavity. Angew. Chem. Int. Ed. Engl. 9, 694–695 (1978)
Gröger, M., Kretzer, K.E., Woyke, A.: Cyclodextrine. Science Forum an der Universität Siegen, Siegen (2001)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998)
Challa, R., Ahuja, A., Ali, J., Khar, R.K.: Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 6(2), 327–359 (2005)
Pinto, L.M.A., Fernandes, F.L., Santana, M.H.A., Pertinhez, T.A., Oyama, S., Junior de Paula, E.: Physico-chemical characterization of benzocaine-β-cyclodextrin inclusion complexes. J. Pharm. Biomed. Anal. 39, 956–963 (2005)
Ingle, J.R., Busch, K.W., Busch, M.A.: Chiral analysis by multivariate regression modeling of spectral data using cyclodextrin guest–host complexes—methods for determining enantiomeric composition with varying chiral analyte concentration. Talanta 75, 572–584 (2008)
Praliev, K.D., Yu, V.K., Poplavskaya, I.A.: Target synthesis of pharmacologically active derivatives of 4-ethynyl-4-hydroxypiperidine. In: Kartsev, V.G., Tolstikov, G.A. (eds.) Nitrogen-Containing Heterocycles and Alcaloides, pp. 484–489. IBS PRESS, Moscow (2001)
Yu, V.K., Nagimova, A.D., Praliev, K.D., Shin, S.N., De Kempe, N.: Synthesis, antibacterial, and analgesic activity of 1-(2-ethoxyethyl)-4-hydroxy(acyloxy)-piperidine-4-carboxylic acids. Pharm. Chem. J. 36(7), 282–284 (2002)
Praliev, K.D., Isin, Zh., Yu, V.K., Tarakov, S.A., Bosyakov, Yu.G., Utepbergenova, R.K., Shin, S.N., Kadyrova, D.M.: Hydrochloride of 1-(2-ethoxyethyl)-4-ethynyl-4-benzoyloxy-piperidine possesses anesthetic activity. Patent Ru. 1704415 (1996)
Siemens Industrial Automation, Inc., SAINT: Area-Detector Integration Software. Madison, WI, (1995)
Siemens Industrial Automation, Inc., SADABS: Area-Detector Absorption Correction. Madison, WI (1995)
Sheldrick, G.M.: A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008)
Allen, F.H., Kennard, O., Watson, G.D., Brammer, L., Orpen, A.G., Taylor, R.: Tables of bonds lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. II, 1–17 (1987)
Harata, K.: Structural aspects of stereodifferentiation in the solid state. Chem. Rev. 98, 1803–1827 (1998)
Mino, R.C., Vivienne, J.G., Luigi, R.N., van Bosch, O.: Synthesis and X-ray crystal structure of β-cyclodextrin diclofenac sodium undecahydrate, a β-CD complex with a unique crystal packing arrangement. J. Chem. Soc. Chem. Commun. 1061–1062 (1994)
Bonnet, P., Jaime, C., Morin-Allory, L.: Structure and thermodynamics of a-, b-, and g-cyclodestrin dimers. Molecular dynamics studies of the solvent effect and free binding energies. J. Org. Chem. 67, 8602–8609 (2002)
Quevauviller, A.: Local Anaesthethics in Experimental Methods for Comparing Local Anaesthetic Activity—International Encyclopedia of Pharmacology and Therapeutics, pp. 291–318. Pergaman Press, Fayetteville (1971)
Dib, B.: Intrathecal chronic cathetarisation in the rat. Pharmacol. Biochem. Behav. 20, 45–48 (1984)
Camougis, G., Takman, B.H.: Methods in Pharmacology. Appleton Century Crofts, New York (1971)
Acknowledgements
The authors acknowledge financial support by Volkswagen Stiftung in the frame of the international focus “Between Europe and the Orient”. We acknowledge help with in-house X-ray data collection by Prof. Ulrich Abram.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kemelbekov, U.S., Hagenbach, A., Lentz, D. et al. Pharmacology and structures of the free base of the anaesthetic kazcaine and its complex with β-cyclodextrin. J Incl Phenom Macrocycl Chem 68, 323–330 (2010). https://doi.org/10.1007/s10847-010-9791-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9791-7