Abstract
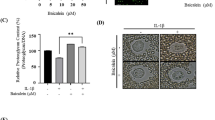
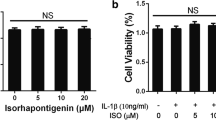
In the present study, we demonstrated the anti-catabolic effects of formononetin, a phytoestrogen derived from herbal plants, against interleukin-1β (IL-1β)-induced severe catabolic effects in primary rat chondrocytes and articular cartilage. Formononetin did not affect the viability of primary rat chondrocytes in both short- (24 h) and long-term (21 days) treatment periods. Furthermore, formononetin effectively antagonized the IL-1β-induced catabolic effects including the decrease in proteoglycan content, suppression of pericellular matrix formation, and loss of proteoglycan through the decreased expression of cartilage-degrading enzymes like matrix metalloproteinase (MMP)-13, MMP-1, and MMP-3 in primary rat chondrocytes. Moreover, catabolic oxidative stress mediators like nitric oxide, inducible nitric oxide synthase, cyclooxygenase-2, and prostaglandin E2 were significantly downregulated by formononetin in primary rat chondrocytes treated with IL-1β. Sequentially, the upregulation of pro-inflammatory cytokines (like IL-1α, IL-1β, IL-6, and tumor necrosis factor α), chemokines (like fractalkine, monocyte chemoattractant protein-1, and macrophage inflammatory protein-3α), and vascular endothelial growth factor were significantly downregulated by formononetin in primary rat chondrocytes treated with IL-1β. These data suggest that formononetin may suppress IL-1β-induced severe catabolic effects and osteoarthritic condition. Furthermore, formononetin may be a promising candidate for the treatment and prevention of osteoarthritis.






Similar content being viewed by others
References
Goldring, M.B., and S.R. Goldring. 2007. Osteoarthritis. Journal of Cellular Physiology 213: 626–634.
Barr, A.J., T.M. Campbell, D. Hopkinson, S.R. Kingsbury, M.A. Bowes, and P.G. Conaghan. 2015. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Research & Therapy 17: 228.
Xie, F., B. Kovic, X. Jin, X. He, M. Wang, and C. Silvestre. 2016. Economic and humanistic burden of osteoarthritis: A systematic review of large sample studies. Pharmacoeconomics 34: 1087–1100.
Chen, D., J. Shen, W. Zhao, T. Wang, L. Han, J.L. Hamilton, and H.J. Im. 2017. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Research 5: 16044.
Zhang, W., H. Ouyang, C.R. Dass, and J. Xu. 2016. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Research 4: 15040.
Luria, A., and C.R. Chu. 2014. Articular cartilage changes in maturing athletes: New targets for joint rejuvenation. Sports Health 6: 18–30.
Kim, H., D. Kang, Y. Cho, and J.H. Kim. 2015. Epigenetic regulation of chondrocyte catabolism and anabolism in osteoarthritis. Molecules and Cells 38: 677–684.
Lee, A.S., M.B. Ellman, D. Yan, J.S. Kroin, B.J. Cole, A.J. van Wijnen, and H.J. Im. 2013. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 527: 440–447.
Akkiraju, H., and A. Nohe. 2015. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. Journal of Developmental Biology 3: 177–192.
Nie, T., S. Zhao, L. Mao, Y. Yang, W. Sun, X. Lin, S. Liu, K. Li, Y. Sun, P. Li, Z. Zhou, S. Lin, X. Hui, A. Xu, C.W. Ma, Y. Xu, C. Wang, P.R. Dunbar, and D. Wu. 2018. The natural compound, formononetin, extracted from Astragalus membranaceus increases adipocyte thermogenesis by modulating PPARgamma activity. British Journal of Pharmacology 175: 1439–1450.
Mu, H., Y.H. Bai, S.T. Wang, Z.M. Zhu, and Y.W. Zhang. 2009. Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover). Phytomedicine 16: 314–319.
Ma, Z., W. Ji, Q. Fu, and S. Ma. 2013. Formononetin inhibited the inflammation of LPS-induced acute lung injury in mice associated with induction of PPAR gamma expression. Inflammation 36: 1560–1566.
Wu, J., X. Ke, N. Ma, W. Wang, W. Fu, H. Zhang, M. Zhao, X. Gao, X. Hao, and Z. Zhang. 2016. Formononetin, an active compound of Astragalus membranaceus (Fisch) Bunge, inhibits hypoxia-induced retinal neovascularization via the HIF-1alpha/VEGF signaling pathway. Drug Design, Development and Therapy 10: 3071–3081.
Xia, B., Chen Di, J. Zhang, S. Hu, H. Jin, and P. Tong. 2014. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcified Tissue International 95: 495–505.
Cameron, M., and S. Chrubasik. 2014. Oral herbal therapies for treating osteoarthritis. Cochrane Database of Systematic Reviews CD002947.
Greene, M.A., and R.F. Loeser. 2015. Aging-related inflammation in osteoarthritis. Osteoarthritis and Cartilage 23: 1966–1971.
Rezuș, E., A. Cardoneanu, A. Burlui, A. Luca, C. Codreanu, B.I. Tamba, G.D. Stanciu, N. Dima, C. Bădescu, and C. Rezuș. 2019. The link between inflammaging and degenerative joint diseases. International Journal of Molecular Sciences: 20.
Nees, T.A., N. Rosshirt, T. Reiner, M. Schiltenwolf, and B. Moradi. 2018. Inflammation and osteoarthritis-related pain. Der Schmerz.
Mathiessen, A., and P.G. Conaghan. 2017. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Research & Therapy 19: 18.
Abramson, S.B. 2008. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Research & Therapy 10: S2.
Notoya, K., D.V. Jovanovic, P. Reboul, J. Martel-Pelletier, F. Mineau, and J.P. Pelletier. 2000. The induction of cell death in human osteoarthritis chondrocytes by nitric oxide is related to the production of prostaglandin E2 via the induction of cyclooxygenase-2. Journal of Immunology 165: 3402–3410.
Park, J.Y., M.H. Pillinger, and S.B. Abramson. 2006. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clinical Immunology 119: 229–240.
Schuerwegh, A.J., E.J. Dombrecht, W.J. Stevens, J.F. Van Offel, C.H. Bridts, and L.S. De Clerck. 2003. Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis and Cartilage 11: 681–687.
Caglic, D., U. Repnik, C. Jedeszko, G. Kosec, C. Miniejew, M. Kindermann, O. Vasiljeva, et al. 2013. The proinflammatory cytokines interleukin-1alpha and tumor necrosis factor alpha promote the expression and secretion of proteolytically active cathepsin S from human chondrocytes. Biological Chemistry 394: 307–316.
Richardson, D.W., and G.R. Dodge. 2000. Effects of interleukin-1beta and tumor necrosis factor-alpha on expression of matrix-related genes by cultured equine articular chondrocytes. American Journal of Veterinary Research 61: 624–630.
Heraud, F., A. Heraud, and M.F. Harmand. 2000. Apoptosis in normal and osteoarthritic human articular cartilage. Annals of the Rheumatic Diseases 59: 959–965.
Mohtai, M., M.K. Gupta, B. Donlon, B. Ellison, J. Cooke, G. Gibbons, D.J. Schurman, and R.L. Smith. 1996. Expression of interleukin-6 in osteoarthritic chondrocytes and effects of fluid-induced shear on this expression in normal human chondrocytes in vitro. Journal of Orthopaedic Research 14: 67–73.
Zhou, R., X. Wu, Z. Wang, J. Ge, and F. Chen. 2015. Interleukin-6 enhances acid-induced apoptosis via upregulating acid-sensing ion channel 1a expression and function in rat articular chondrocytes. International Immunopharmacology 29: 748–760.
Sandell, L.J., X. Xing, C. Franz, S. Davies, L.W. Chang, and D. Patra. 2008. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthritis and Cartilage 16: 1560–1571.
Zou, Y., Y. Li, L. Lu, Y. Lin, W. Liang, Z. Su, X. Wang, H. Yang, J. Wang, C. Yu, L. Huo, and Y. Ye. 2013. Correlation of fractalkine concentrations in serum and synovial fluid with the radiographic severity of knee osteoarthritis. Annals of Clinical Biochemistry 50: 571–575.
Huo, L.W., Y.L. Ye, G.W. Wang, and Y.G. Ye. 2015. Fractalkine (CX3CL1): A biomarker reflecting symptomatic severity in patients with knee osteoarthritis. Journal of Investigative Medicine 63: 626–631.
Klosowska, K., M.V. Volin, N. Huynh, K.K. Chong, M.M. Halloran, and J.M. Woods. 2009. Fractalkine functions as a chemoattractant for osteoarthritis synovial fibroblasts and stimulates phosphorylation of mitogen-activated protein kinases and Akt. Clinical & Experimental Immunology 156: 312–319.
Wojdasiewicz, P., L.A. Poniatowski, A. Kotela, J. Deszczynski, I. Kotela, and D. Szukiewicz. 2014. The chemokine CX3CL1 (fractalkine) and its receptor CX3CR1: Occurrence and potential role in osteoarthritis. Archivum Immunologiae et Therapiae Experimentalis 62: 395–403.
Xu, Y.K., Y. Ke, B. Wang, and J.H. Lin. 2015. The role of MCP-1-CCR2 ligand-receptor axis in chondrocyte degradation and disease progress in knee osteoarthritis. Biological Research 48: 64.
Alaaeddine, N., J. Antoniou, M. Moussa, G. Hilal, G. Kreichaty, I. Ghanem, W. Abouchedid, E. Saghbini, and J.A. Di Battista. 2015. The chemokine CCL20 induces proinflammatory and matrix degradative responses in cartilage. Inflammation Research 64: 721–731.
Lingaraj, K., C.K. Poh, and W. Wang. 2010. Vascular endothelial growth factor (VEGF) is expressed during articular cartilage growth and re-expressed in osteoarthritis. Annals of the Academy of Medicine, Singapore 39: 399–403.
Zhang, X., R. Crawford, and Y. Xiao. 2016. Inhibition of vascular endothelial growth factor with shRNA in chondrocytes ameliorates osteoarthritis. Journal of Molecular Medicine 94: 787–798.
Barranco, C. 2014. Osteoarthritis: Animal data show VEGF blocker inhibits post-traumatic OA. Nature Reviews Rheumatology 10: 638.
Miller, R.E., R.J. Miller, and A.M. Malfait. 2014. Osteoarthritis joint pain: The cytokine connection. Cytokine 70: 185–193.
Hamilton, J.L., M. Nagao, B.R. Levine, D. Chen, B.R. Olsen, and H.J. Im. 2016. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. Journal of Bone and Mineral Research 31: 911–924.
Acknowledgments
This study was supported by research fund from Chosun University, 2017.
Author information
Authors and Affiliations
Contributions
I.A.C, T.H.K., K.R.K, H.L., J.H.P., S.Y.L., and J.S.K. contributed to the experimental design and collected the data. C.S.K., D.K.K., H.K.K., S.K.Y., S.G.K., and J.S.K. contributed to the data analysis and interpretation. I.A.C., K.R.K., and J.S.K. did the writing article.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cho, IA., Kim, TH., Lim, H. et al. Formononetin Antagonizes the Interleukin-1β-Induced Catabolic Effects Through Suppressing Inflammation in Primary Rat Chondrocytes. Inflammation 42, 1426–1440 (2019). https://doi.org/10.1007/s10753-019-01005-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01005-1