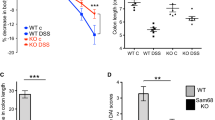
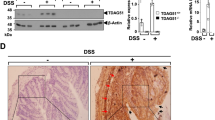
Abstract
Introduction
OPN has been implicated in the inflammatory response to Crohn’s disease. We hypothesized that OPN deficiency protects against different stages of TNBS-induced colitis in a modified model that mimics Crohn’s disease.
Material and Methods
OPN-deficient and wildtype mice were treated intracolonically with TNBS and euthanized during acute, sub-acute and chronic colitis.
Results
TNBS-treated wildtype mice developed severe colitis, but OPN-deficient mice were significantly protected. Wildtype mice showed significant infiltration of inflammatory cells including macrophages, and colonic transmural thickening that progressed to strictures, increased matrix collagen deposits (X2 fold), and granuloma formation. These pathological findings were partially attenuated by OPN deficiency. The inflammatory marker, serum amyloid A (SAA), markedly increased in sub-acute stages regardless of OPN status. Conversely, OPN deficiency significantly reduced concentration of SAA in the acute and chronic stages. Secretory OPN was upregulated particularly in acute stage in wildtypes (P < 0.001) and as expected not present in OPN-deficient animals. Flow cytometry analysis of splenic macrophages revealed significant increases in scavenger receptors, macrosialin and F4/80 markers’ expression in wildtypes.
Conclusions
Our data support the role of OPN in induction of inflammation and establishment of chronic colitis. Therefore, OPN may represent a target for therapeutic intervention in Crohn’s disease.







Similar content being viewed by others
Abbreviations
- DSS:
-
Dextran sodium sulfate
- FITC:
-
Fluorescein isothiocyanate
- IBD:
-
Inflammatory bowel disease
- OPN:
-
Osteopontin
- PE:
-
Phycoerythrin, labeled
- SAA:
-
Serum amyloid A
- SR-A:
-
Scavenger receptor class A
- TNBS:
-
Trinitrobenzene sulfonic acid
- WT:
-
Wildtype
References
Caillier S, Barcellos LF, Baranzini SE, et al. Multiple sclerosis genetics group. Osteopontin polymorphisms and disease course in multiple sclerosis. Genes Immun. 2003;4:312–315.
Sato T, Nakai T, Tamura N, et al. Osteopontin/Eta-1 upregulated in Crohn’s disease regulates the Th1 immune response. Gut. 2005;54:1254–1262.
Mishima R, Takeshima F, Sawai T, et al. High plasma osteopontin levels in patients with inflammatory bowel disease. J Clin Gastroenterol. 2007;41:123–125.
Oz HS, Ebersole JL. Application of prodrugs to inflammatory diseases of the gut. Molecules. 2008;13:452–474 (review).
Neuman MG. Immune dysfunction in inflammatory bowel disease. Transl Res. 2007;149:173–186.
Hume DA, Allan W, Hogan PG, Doe WF. Immunohistochemical characterization of macrophages in human liver and gastrointestinal tract: expression of CD4, HLA-DR, OKM1, and the mature macrophage marker 25F9 in normal and diseased tissue. J Leuko Biol. 1987;42:474–484.
Oshitani N, Campbell A, Kitano A, Kobayashi K, Jewell DP. In situ comparison of phenotypical and functional activity of infiltrating cells in ulcerative colitis mucosa. J Pathol. 1996;178:95–99.
Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35:669–674.
Grimm MC, Pullman WE, Bennett GM, Sullivan PJ, Pavli P, Doe WF. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J Gastro Hepatol. 1995;10:387–395.
Patarca R, Wei FY, Singh P, Morasso MI, Cantor H. Dysregulated expression of the T cell cytokine Eta-1 in CD4–8-lymphocytes during the development of murine autoimmune disease. J Exp Med. 1990;172:1177–1183.
Lampe MA, Patarca R, Iregui MV, Cantor H. Polyclonal B cell activation by the Eta-1 cytokine and the development of systemic autoimmune disease. J Immunol. 1991;147:2902–2906.
Yamamoto N, Sakai F, Kon S, et al. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clinic Invest. 2003;112:181–188.
Masuda H, Takahashi Y, Asai S, Hemmi A, Takayama T. Osteopontin expression in ulcerative colitis is distinctly different from that in Crohn’s disease and diverticulitis. J Gastro. 2005;40:409–413.
Oz HS, Chen T, Nagasawa H. Comparative efficacies of two cysteine prodrugs and a glutathione delivery agent in a colitis model. Transl Res. 2007;150:122–129.
Zhong J, Eckhardt ER, Oz HS, Bruemmer D, de Villiers W. Osteopontin deficiency protects mice from DSS-induced colitis. Inflam Bowel Dis. 2006;12:790–796.
Oz HS, Chen T, Ebersole JL. A model for chronic mucosal inflammation in IBD and periodontitis. Dig Dis Sci. 2010;55:2194–2202.
Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin—a possible anchor of osteoclasts to bone. Proc Natl Acad Sci USA. 1990;87:4473–4475.
Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–622.
Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864.
Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–358.
Heilmann K, Hoffmann U, Witte E, et al. Osteopontin as two-sided mediator of intestinal inflammation. J Cell Mol Med. 2009;13:1162–1174.
Da Silva AP, Pollett A, Rittling SR, Denhardt DT, Sodek J, Zohar R. Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-alpha expression and non-programmed cell death. J Cell Physiol. 2006;208:629–639.
Grassl GA, Valdez Y, Bergstrom KS, Vallance BA, Finlay BB. Chronic enteric salmonella infection in mice leads to severe and persistent intestinal fibrosis. Gastroenterology. 2008;134:768–780.
Ardite E, Sans M, Panes J, Romero F, Pique JM, Fernandez-Checa J. Replenishment of glutathione levels improves mucosal function in experimental acute colitis. Lab Invest. 2000;80:735–744.
Oz HS, Ebersole JL. A novel murine model for chronic inflammatory alveolar bone loss. J Periodontal Resh. 2010;45:94–99.
Grisham MB, Volkmer C, Tso P, Yamada T. Metabolism of trinitrobenzene sulfonic acid by the rat colon produces reactive oxygen species. Gastroenterology. 1991;101:540–547.
Rath HC, Schultz M, Freitag R, et al. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect Immun. 2001;69:2277–2285.
Hazelgrove KB, Flynn RS, Qiao lY, Grider JR, Kuemmerle JF. Endogenous IGF-I and αvβ3 integrin ligands regulate increased smooth muscle growth in TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1230–G1237.
Acknowledgments
This research was partially supported by the National Institutes of Health grants: NCCAM-AT1490 and NIDCR-DE19177 (HO). This study was presented in part as a poster of distinction at the Digestive Disease Week 2008 in San Diego, CA (Gastroenterology Suppl 2008:134,A-525,T1296).
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oz, H.S., Zhong, J. & de Villiers, W.J.S. Osteopontin Ablation Attenuates Progression of Colitis in TNBS Model. Dig Dis Sci 57, 1554–1561 (2012). https://doi.org/10.1007/s10620-011-2009-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-2009-z