Abstract
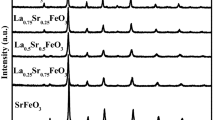
In this paper a wide range of La1−x Sr x MnO3 (x = 0–0.7) perovskites was synthesized by Pechini route, characterized by XRD (including high temperature measurements), XPS, differential dissolution phase analysis, TPR H2, oxygen exchange and tested in N2O decomposition at 900 °C. At low degree of Sr substitution for La (x ≤ 0.3), high catalytic activity was found for perovskites with hexagonal structure (x = 0.1–0.2) and can be related to fast oxygen mobility caused by the lattice disordering during polymorphic phase transition from the hexagonal to cubic structure. For multiphase samples (x > 0.3) increase of activity and oxygen mobility can be attributed to the formation of the layer-structured perovskite–LaSrMnO4 on the surface.
Graphical Abstract
Catalytic activity of La–Sr–Mn–O samples in high-temperature N2O decomposition is discussed with emphasis on its relation to oxygen mobility, surface composition and microstructure of the catalyst particles.









Similar content being viewed by others
Abbreviations
- DDPA:
-
Differential dissolution phase analysis
References
Ivanov DV, Sadovskaya EM, Pinaeva LG, Isupova LA (2009) J Catal 267(1):5–13
Yamazoe N, Teraoka Y (1990) Catal Today 8:175–179
Seyama T (1993) In: Tejuca LG, Fierro JLG (eds) Properties and application of perovskite-type oxides. Marcel Dekker, New York
Ponce S, Pena MA, Fierro JLG (2000) Appl Catal B: Environ 24:193–205
O’Connel M, Norman AK, Huttermann CF, Morris MA (1999) Catal Today 47:123–132
Kinner SJS, Sayers R, Grimes RW (2005) In: Stammes N et al (eds) Full cell technologies: state and perspectives. Springer, Netherlands
Sase M, Yashiro K, Sato K, Mizusaki J, Kawada T, Sakai N, Yamaji K, Horita T, Yokokawa H (2008) Solid State Ionics 178:1843–1852
Pechini MP (1967) US Patent no. 3,330,697
Karita R, Kusaba H, Sasaki K, Teraoka Y (2007) Catal Today 126:471–475
Malakhov VV (2000) J Mol Catal A 158:143–148
Berger RJ, Kapteijn F, Moulijn JA, Marin GB, Wilde JD, Olea M, Chen D, Holmen A, Lietti L, Tronconi E, Schuurman Y (2008) Appl Catal A: Gen 342:3–28
Mizutani N, Kitazawa A, Ohkuma N, Kato M (1970) J Chem Soc (Japan) Ind Ed 73:1097–1103
Cherepanov VA, Barkhatova LYu, Voronin VL (1997) J Solid State Chem 134:38–44
Majewski P, Epple L, Rozumek M, Schluckwerder H, Aldiner F (2000) J Mater Res 15(5):1161–1166
Rormark L, Wiik K, Stolen S, Grande T (2002) J Mater Chem 12:1058–1067
Atsumi T, Kamegashira N (1997) J Alloys Compd 257:161–167
Andrieux M, Picard C (2000) J Mater Sci Lett 19:695–697
Kapteijn F, Rodriguez–Mirasol J, Moulijn J (1996) Appl Catal B: Environ 9:25–64
Sazonov LA, Mosvina ZV, Artamonov EV (1970) Kinet Katal 15:120
Winter ERS (1968) J Chem Soc A 12:2889
Raj SL, Srinivasan V (1980) J Cat 65(1):121
Winter ERS (1974) J Catal 34:431–439
Dulli H, Dowben PA, Liou SH, Plummer EW (2000) Phys Rev B 62(22):R14629
Hirsch PH, Howie A, Nicholson RB, Pashley DW, Whelan MJ (1965) Electron microscopy of thin crystal. Butterworths, London
Isupova LA, Nadeev AN, Yakovleva IS, Tsybulya SV (2007) Kinet Catal 49(1):142–146
Vogt T, Schmahl WW (1993) Europhys Lett 24(4):281–285
Goodenough JB (2003) In: Gschneidner KA Jr, Bunzli J-CG, Pecharsky VK (eds) Handbook on the physics and chemistry of rare earth, vol 33. Elsevier Science B.V., North-Holland
Li RK, Gleaves C (2000) J Solid State Chem 153:34–40
Kharton VV, Sobyanin VA, Belyaev VA, Semin GL, Veniaminov SV, Tsipis EV, Yaremchenko AA, Valente AA, Marozau IP, Frade JR, Rocha J (2004) Catal Commun 5(6):311–316
Yakovleva IS, Isupova LA, Rogov VA, Sadykov VA (2008) Kinet Catal 49:274–283
Ciambelli P, Cimino S, De Rossi S, Faticanti M, Lisi L, Minelli G, Pettiti I, Porta P, Russo G, Turko M (2000) Appl Catal B: Environ 24:243
Swamy CS, Christopher J (1992) Catal Rev Sci Eng 34(4):409
Acknowledgments
We would like to acknowledge the contribution of Dr. Ekaterina Sadovskaya for modeling of oxygen exchange, Prof. Sergey Tsybulya for fruitful discussion, Mrs. Nina Kulikovskaya for assistance in samples preparation and Mr. Eugene Gerasimov for XRD measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ivanov, D.V., Pinaeva, L.G., Isupova, L.A. et al. Insights into the Reactivity of La1−x Sr x MnO3 (x = 0 ÷ 0.7) in High Temperature N2O Decomposition. Catal Lett 141, 322–331 (2011). https://doi.org/10.1007/s10562-010-0503-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0503-0