Abstract
Purpose
The prolactin (PRL) receptor is over-expressed in breast cancer, and pre-clinical data indicate that it contributes to breast oncogenesis. Cabergoline is a potent dopamine receptor agonist of D2 receptors and has a direct inhibitory effect on pituitary PRL secretion.
Methods
A phase II study of cabergoline in patients with metastatic breast cancer was conducted. The primary end point of the study was to determine the clinical benefit rate (CBR) at 2 months. Eligible patients had tumors of any receptor status with no limit of prior lines of therapy. Measurable and unmeasurable diseases were allowed. Cabergoline 1 mg orally, twice weekly (1 cycle = 4 weeks) was given until disease progression or unacceptable toxicity. PRL receptor immunohistochemical staining was performed on available baseline tumor tissue; serial serum PRL levels were assessed.
Results
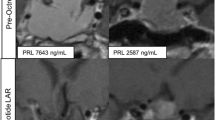
Twenty women were enrolled; 18 were evaluable for CBR. Tumor receptor status was distributed as follows: HR−any/HER2+ 2(10%), HR+/HER2− 18 (90%). The CBR was 33% (6/18), median progression free survival was 1.8 months, and median overall survival was 10.4 months. Two patients experienced disease control for over 12 months. Most common treatment-related adverse events were nausea (30%), fatigue (25%), and elevation in alkaline phosphatase (15%). Nine patients had baseline tissue for analysis; there was no association between baseline tumor PRL receptor expression and clinical benefit (p = 0.24). Change in serum PRL level and response were not correlated after 2 months of treatment (p = 0.64).
Conclusion
Cabergoline was well tolerated, and while the ORR was low, a small subset of patients experienced extended disease control.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics. CA Cancer J Clin 66(1):7–30. doi:10.3322/caac.21332
Baselga J, Campone M, Piccart M, Burris HA III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366(6):520–529. doi:10.1056/NEJMoa1109653
Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, Giorgetti C, Randolph S, Koehler M, Cristofanilli M M, Group PS (2015) Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373(3):209–219. doi:10.1056/NEJMoa1505270
Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, Verhoeven D, Pedrini JL, Smirnova I, Lichinitser MR, Pendergrass K, Garnett S, Lindemann JP, Sapunar F, Martin M (2010) Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 28(30):4594–4600. doi:10.1200/JCO.2010.28.8415
Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM, Group CS (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366(2):109–119. doi:10.1056/NEJMoa1113216
Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Dieras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C, Investigators E (2011) Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377(9769):914–923. doi:10.1016/S0140-6736(11)60070-6
Clevenger CV, Furth PA, Hankinson SE, Schuler LA (2003) The role of prolactin in mammary carcinoma. Endocr Rev 24(1):1–27. doi:10.1210/er.2001-0036
Clevenger CV, Chang WP, Ngo W, Pasha TL, Montone KT, Tomaszewski JE (1995) Expression of prolactin and prolactin receptor in human breast carcinoma. Evidence for an autocrine/paracrine loop. Am J Pathol 146(3):695–705
Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA (2003) Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene 22(30):4664–4674. doi:10.1038/sj.onc.1206619
Holtkamp W, Nagel GA, Wander HE, Rauschecker HF, von Heyden D (1984) Hyperprolactinemia is an indicator of progressive disease and poor prognosis in advanced breast cancer. Int J Cancer 34(3):323–328
Cancer Genome Atlas N (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70. doi:10.1038/nature11412
Welsch CW, Nagasawa H (1977) Prolactin and murine mammary tumorigenesis: a review. Cancer Res 37(4):951–963
Bonneterre J, Mauriac L, Weber B, Roche H, Fargeot P, Tubiana-Hulin M, Sevin M, Chollet P, Cappelaere P (1988) Tamoxifen plus bromocriptine versus tamoxifen plus placebo in advanced breast cancer: results of a double blind multicentre clinical trial. Eur J Cancer Clin Oncol 24(12):1851–1853
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Thomas ES, Gomez HL, Li RK, Chung HC, Fein LE, Chan VF, Jassem J, Pivot XB, Klimovsky JV, de Mendoza FH, Xu B, Campone M, Lerzo GL, Peck RA, Mukhopadhyay P, Vahdat LT, Roche HH (2007) Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 25(33):5210–5217. doi:10.1200/JCO.2007.12.6557
Keller AM, Mennel RG, Georgoulias VA, Nabholtz JM, Erazo A, Lluch A, Vogel CL, Kaufmann M, von Minckwitz G, Henderson IC, Mellars L, Alland L, Tendler C (2004) Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J Clin Oncol 22(19):3893–3901. doi:10.1200/JCO.2004.08.157
Ingle JN, Suman VJ, Rowland KM, Mirchandani D, Bernath AM, Camoriano JK, Fishkin PA, Nikcevich DA, Perez EA, North Central Cancer Treatment Group Trial N (2006) Fulvestrant in women with advanced breast cancer after progression on prior aromatase inhibitor therapy: North Central Cancer Treatment Group Trial N0032. J Clin Oncol 24(7):1052–1056. doi:10.1200/JCO.2005.04.1053
Bines J, Dienstmann R, Obadia RM, Branco LG, Quintella DC, Castro TM, Camacho PG, Soares FA, Costa ME (2014) Activity of megestrol acetate in postmenopausal women with advanced breast cancer after nonsteroidal aromatase inhibitor failure: a phase II trial. Ann Oncol 25(4):831–836. doi:10.1093/annonc/mdu015
Catania C, Ascione G, Adamoli L, De Pas T, Medici M, Franceschelli L, Verri E, Magni E, Sanna G, Torrisi R, Goldhirsch A, Nole F (2007) Fulvestrant in heavily pre-treated patients with advanced breast cancer: results from a single compassionate use programme centre. Breast Cancer Res Treat 106(1):97–103. doi:10.1007/s10549-006-9481-8
Lonning PE, Taylor PD, Anker G, Iddon J, Wie L, Jorgensen LM, Mella O, Howell A (2001) High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat 67(2):111–116
Agarwal N, Machiels JP, Suarez C, Lewis N, Higgins M, Wisinski K, Awada A, Maur M, Stein M, Hwang A, Mosher R, Wasserman E, Wu G, Zhang H, Zieba R, Elmeliegy M (2016) Phase I study of the prolactin receptor antagonist LFA102 in metastatic breast and castration-resistant prostate cancer. Oncologist 21(5):535–536. doi:10.1634/theoncologist.2015-0502
Krysiak R, Okopien B (2015) Different effects of cabergoline and bromocriptine on metabolic and cardiovascular risk factors in patients with elevated prolactin levels. Basic Clin Pharmacol Toxicol 116(3):251–256. doi:10.1111/bcpt.12307
Frontini L, Lissoni P, Vaghi M, Perego MS, Pescia S, Ardizzoia A, Gardani G (2004) Enhancement of the efficacy of weekly low-dose taxotere by the long acting anti-prolactinemic drug cabergoline in pretreated metastatic breast cancer. Anticancer Res 24(6):4223–4226
Scotti ML, Langenheim JF, Tomblyn S, Springs AE, Chen WY (2008) Additive effects of a prolactin receptor antagonist, G129R, and herceptin on inhibition of HER2-overexpressing breast cancer cells. Breast Cancer Res Treat 111(2):241–250. doi:10.1007/s10549-007-9789-z
Rasmussen LM, Frederiksen KS, Din N, Galsgaard E, Christensen L, Berchtold MW, Panina S (2010) Prolactin and oestrogen synergistically regulate gene expression and proliferation of breast cancer cells. Endocr Relat Cancer 17(3):809–822. doi:10.1677/ERC-09-0326
Gonzalez L, Zambrano A, Lazaro-Trueba I, Lopez E, Gonzalez JJ, Martin-Perez J, Aranda A (2009) Activation of the unliganded estrogen receptor by prolactin in breast cancer cells. Oncogene 28(10):1298–1308. doi:10.1038/onc.2008.473
Funding
This study was supported by Lynn Sage Breast Cancer Research Foundation, Dolores Knes Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No relevant disclosures from any authors.
Rights and permissions
About this article
Cite this article
Costa, R., Santa-Maria, C.A., Scholtens, D.M. et al. A pilot study of cabergoline for the treatment of metastatic breast cancer. Breast Cancer Res Treat 165, 585–592 (2017). https://doi.org/10.1007/s10549-017-4370-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4370-x