Abstract
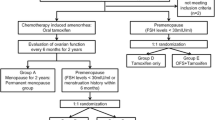
The purpose of this randomized study was to examine if goserelin concomitant to CMF-chemotherapy as adjuvant treatment for premenopausal breast cancer, protects the ovaries from premature failure. A total of 285 premenopausal breast cancer patients, in a randomized adjuvant trial (Zoladex in premenopausal patients (ZIPP)), were assigned to a study on ovarian function. Node positive patients were assigned to CMF-(cyclophosphamide, methotrexate and 5-fluorouracil) chemotherapy in addition to endocrine therapy. All patients were randomly assigned to receive 2 years of goserelin, goserelin plus tamoxifen, tamoxifen alone or no endocrine treatment. We studied, if menses were affected in the treatment groups, up to 36 months after randomization. One year after completed CMF- and endocrine therapy, 36% of the women in the goserelin group reported menses, compared to 7% in the goserelin plus tamoxifen group, 13% in the tamoxifen group and 10% of the controls. Among women treated with goserelin, there was a statistically significant increase in the proportion of menstruating women, 1 year after completed treatment compared to at 24 months of treatment (P = 0.006), in contrast to all other treatment groups, who were unchanged or more often amenorrheic. In our study, there is some evidence of protective effect of goserelin on ovarian function in CMF treated women. This effect was not observed in the combined tamoxifen and goserelin treatment.



Similar content being viewed by others
References
Bines J, Oleske DM, Cobleigh MA (1996) Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol 14(5):1718–1729
Bernhard J et al (2007) Adjuvant chemotherapy followed by goserelin compared with either modality alone: the impact on amenorrhea, hot flashes, and quality of life in premenopausal patients—the International Breast Cancer Study Group Trial VIII. J Clin Oncol 25(3):263–270. doi:10.1200/JCO.2005.04.5393
Jonat W et al (2002) Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: the Zoladex Early Breast Cancer Research Association Study. J Clin Oncol 20(24):4628–4635
Goodwin PJ et al (1999) Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 17(8):2365–2370
Padmanabhan N, Rubens RD, Howell A (1986) Adjuvant chemotherapy in early breast cancer. Lancet 328(8519):1333–1334. doi:10.1016/S0140-6736(86)91459-5
Oktay K et al (2003) Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod 18(1):90–95. doi:10.1093/humrep/deg045
Jemal A et al (2006) Cancer statistics, 2006. CA Cancer J Clin 56(2):106–130
Socialstyrelsen (2005) Cancer incidence in Sweden 2005, Stockholm
von Schoultz E et al (1995) Influence of prior and subsequent pregnancy on breast cancer prognosis. J Clin Oncol 13(2):430–434
Ives A et al (2007) Pregnancy after breast cancer: population based study. BMJ 334(7586):194. doi:10.1136/bmj.39035.667176.55
Goldhirsch A et al (2005) Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 16(10):1569–1583. doi:10.1093/annonc/mdi326
Shapiro CL, Manola J, Leboff M (2001) Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol 19(14):3306–3311
Fornier MN et al (2005) Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer 104(8):1575–1579. doi:10.1002/cncr.21385
Martin M et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352(22):2302–2313. doi:10.1056/NEJMoa043681
Citron ML et al (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21(8):1431–1439. doi:10.1200/JCO.2003.09.081
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
Albain K, Green SJ, Ravdin PM et al (2002) Adjuvant chemohormonal therapy for primary breast cancer should be sequential instead of concurrent: initial results from Intergroup Trial 0100 (SWOG-8814). Proc Am Soc Clin Oncol 21 (abstr 143)
LHRH-agonists in Early Breast Cancer Overview Group (2007) Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet 369(9574):1711–1723. doi:10.1016/S0140-6736(07)60778-8
Chapman RM, Sutcliffe SB, Malpas JS (1979) Cytotoxic-induced ovarian failure in Hodgkin’s disease. II. Effects on sexual function. JAMA 242(17):1882–1884. doi:10.1001/jama.242.17.1882
Nystedt M et al (2003) Side effects of adjuvant endocrine treatment in premenopausal breast cancer patients: a prospective randomized study. J Clin Oncol 21(9):1836–1844. doi:10.1200/JCO.2003.04.024
Sverrisdottir A et al (2004) Bone mineral density among premenopausal women with early breast cancer in a randomized trial of adjuvant endocrine therapy. J Clin Oncol 22(18):3694–3699. doi:10.1200/JCO.2004.08.148
Cwikel J, Gidron Y, Sheiner E (2004) Psychological interactions with infertility among women. Eur J Obstet Gynecol Reprod Biol 117(2):126–131. doi:10.1016/j.ejogrb.2004.05.004
Chapman RM, Sutcliffe SB, Malpas JS (1979) Cytotoxic-induced ovarian failure in women with Hodgkin’s disease. I. Hormone function. JAMA 242(17):1877–1881. doi:10.1001/jama.242.17.1877
Behringer K et al (2005) Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin’s Lymphoma Study Group. J Clin Oncol 23(30):7555–7564. doi:10.1200/JCO.2005.08.138
Recchia F et al (2002) Goserelin as ovarian protection in the adjuvant treatment of premenopausal breast cancer: a phase II pilot study. Anticancer Drugs 13(4):417–424. doi:10.1097/00001813-200204000-00011
Blumenfeld Z et al (2002) Fertility after treatment for Hodgkin’s disease. Ann Oncol 13(Suppl 1):138–147
Baum M et al (2006) Adjuvant goserelin in pre-menopausal patients with early breast cancer: results from the ZIPP study. Eur J Cancer 42(7):895–904. doi:10.1016/j.ejca.2005.12.013
Mardesic T et al (2004) Protocol combining GnRH agonists and GnRH antagonists for rapid suppression and prevention of gonadal damage during cytotoxic therapy. Eur J Gynaecol Oncol 25(1):90–92
Urruticoechea A et al (2008) Ovarian protection with goserelin during adjuvant chemotherapy for pre-menopausal women with early breast cancer (EBC). Breast Cancer Res Treat 110(3):411–416
Potolog-Nahari C, Fishman A, Cohen I (2007) Protection of ovarian function and fertility using a combination of gonadotropin-releasing hormone (GnRH) agonist and GnRH antagonist during cancer treatment in young females. Gynecol Endocrinol 23(5):290–294. doi:10.1080/09513590701327661
Franke HR, Smit WM, Vermes I (2005) Gonadal protection by a gonadotropin-releasing hormone agonist depot in young women with Hodgkin’s disease undergoing chemotherapy. Gynecol Endocrinol 20(5):274–278. doi:10.1080/09513590400027414
Rody A et al (2005) Use of goserelin in the treatment of breast cancer. Expert Rev Anticancer Ther 5(4):591–604. doi:10.1586/14737140.5.4.591
Pacheco B et al (2001) Use of GnRH analogs for functional protection of the ovary and preservation of fertility during cancer treatment in adolescents: a preliminary report. Gynecol Oncol 81(3):391–397. doi:10.1006/gyno.2001.6181
Walshe JM, Denduluri N, Swain SM (2006) Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol 24(36):5769–5779. doi:10.1200/JCO.2006.07.2793
Anderson RA et al (2006) The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod 21(10):2583–2592. doi:10.1093/humrep/del201
Poikonen P et al (2000) Prognostic effect of amenorrhoea and elevated serum gonadotropin levels induced by adjuvant chemotherapy in premenopausal node-positive breast cancer patients. Eur J Cancer 36(1):43–48. doi:10.1016/S0959-8049(99)00225-7
Robertson JF et al (1989) Combined endocrine effects of LHRH agonist (Zoladex) and tamoxifen (Nolvadex) therapy in premenopausal women with breast cancer. Br J Surg 76(12):1262–1265. doi:10.1002/bjs.1800761213
Rossi E et al (2008) Endocrine effects of adjuvant letrozole + triptorelin compared with tamoxifen + triptorelin in premenopausal patients with early breast cancer. J Clin Oncol 26(2):264–270. doi:10.1200/JCO.2007.13.5319
Bonadonna G et al (2005) 30 years’ follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. BMJ 330(7485):217. doi:10.1136/bmj.38314.622095.8F
Acknowledgements
We thank nursing staff at the Breast cancer units, Department of Oncology, Karolinska Hospital for their excellent work coordinating patients’ visits, tests and questionnaires. This study was supported by the Swedish Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sverrisdottir, A., Nystedt, M., Johansson, H. et al. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat 117, 561–567 (2009). https://doi.org/10.1007/s10549-009-0313-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0313-5