Abstract
Background
Mucopolysaccharidosis I (MPS I) is a metabolic disorder caused by α-L-Iduronidase (IDUA) deficiency, resulting in lysosomal accumulation of heparan (HS) and dermatan sulphate (DS). This has been reported in microglia, yet currently the effect of IDUA deficiency on T cells and dendritic cells (DC) and their functionality in disease pathogenesis remains unclear.
Methods
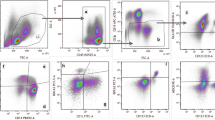
Peripheral blood was collected from 3 month old C57BL/6 MPS I (n = 11) and wildtype (WT) (n = 6) mice. T cell and DC phenotype and functional characteristics were identified by flow cytometry.
Results
MPS I mice exhibited a reduction in DC (p = <0.001) along with CD8+ cytotoxic (p = 0.01) and CD4+ T helper (p = 0.032) cells, compared to WT controls. MPS I DC displayed a significant decrease in cell surface CD123 (p = 0.02) and CD86 (p = 0.006) expression. Furthermore, CD45RB expression was significantly reduced on T helper cells in the MPS I population (p = 0.019).
Conclusion
We report a reduction in circulating DC and T cells in the MPS I mouse; indicative of adaptive immune dysfunction. DC reduction may occur in response to down-regulation of the IL-3 receptor (CD123), necessary for DC survival. We also report down-regulation of cell surface CD86, a molecule required for T cell co-stimulation. T helper cell down-regulation of CD45RB is redolent of an anti-inflammatory phenotype with poor proliferative capacity. The definitive causes of our findings and the consequences and role that these findings play in the pathogenesis of MPS are unclear, but may be in response to lysosomal storage of unmetabolized HS and DS.


Similar content being viewed by others
References
Andersen MH, Schrama D et al (2006) Cytotoxic T cells. J Invest Dermatol 126(1):32–41
Annacker O, Burlen-Defranoux O et al (2000) Regulatory CD4 T cells control the size of the peripheral activated/memory CD4 T cell compartment. J Immunol 164(7):3573–3580
Ausseil J, Desmaris N et al (2008) Early neurodegeneration progresses independently of microglial activation by heparan sulfate in the brain of mucopolysaccharidosis IIIB mice. PLoS One 3(5):e2296
Beissert S, Schwarz A et al (2006) Regulatory T cells. J Invest Dermatol 126(1):15–24
Bossi G, Griffiths GM (1999) Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med 5(1):90–96
Boya P, Gonzalez-Polo RA et al (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25(3):1025–1040
Burman C, Ktistakis NT (2010)Autophagosome formation in mammalian cells. Semin Immunopathol 32(4):397–413
Castaneda JA, Lim MJ et al (2008) Immune system irregularities in lysosomal storage disorders. Acta Neuropathol 115(2):159–174
Clark R, Kupper T (2005) Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol 125(4):629–637
Clarke LA, Russell CS et al (1997) Murine mucopolysaccharidosis type I: targeted disruption of the murine alpha-L-iduronidase gene. Hum Mol Genet 6(4):503–511
Constantopoulos G, Dekaban AS (1978) Neurochemistry of the mucopolysaccharidoses: brain lipids and lysosomal enzymes in patients with four types of mucopolysaccharidosis and in normal controls. J Neurochem 30(5):965–973
Curtsinger JM, Schmidt CS et al (1999) Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol 162(6):3256–3262
Dahlen E, Hedlund G et al (2000) Low CD86 expression in the nonobese diabetic mouse results in the impairment of both T cell activation and CTLA-4 up-regulation. J Immunol 164(5):2444–2456
Dani A, Chaudhry A et al (2004) The pathway for MHCII-mediated presentation of endogenous proteins involves peptide transport to the endo-lysosomal compartment. J Cell Sci 117(Pt 18):4219–4230
Davies JD, O’Connor E et al (1999) CD4+ CD45RB low-density cells from untreated mice prevent acute allograft rejection. J Immunol 163(10):5353–5357
de Groot RP, Coffer PJ et al (1998) Regulation of proliferation, differentiation and survival by the IL-3/IL-5/GM-CSF receptor family. Cell Signal 10(9):619–628
Eskelinen EL, Saftig P (2009) Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta 1793(4):664–673
Fleischer J, Soeth E et al (1996) Differential expression and function of CD80 (B7-1) and CD86 (B7-2) on human peripheral blood monocytes. Immunology 89(4):592–598
Gimmi CD, Freeman GJ et al (1993) Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci U S A 90(14):6586–6590
Groux H, O’Garra A et al (1997) A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389(6652):737–742
Guermonprez P, Valladeau J et al (2002) Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol 20:621–667
Hara M, Kingsley CI et al (2001) IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol 166(6):3789–3796
Harris NL, Ronchese F (1999) The role of B7 costimulation in T-cell immunity. Immunol Cell Biol 77(4):304–311
Holley RJ, Deligny A et al (2011) Mucopolysaccharidosis type I, unique structure of accumulated heparan sulfate and increased N-sulfotransferase activity in mice lacking alpha-l-iduronidase. J Biol Chem 286(43): 37515-24
Joffre O, Nolte MA et al (2009) Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev 227(1):234–247
Johnson DE (1998) Regulation of survival pathways by IL-3 and induction of apoptosis following IL-3 withdrawal. Front Biosci 3:d313–d324
Lim B, Sutherland RM et al (2006) Targeting CD45RB alters T cell migration and delays viral clearance. Int Immunol 18(2):291–300
Luke PP, Deng JP et al (2006) Prolongation of allograft survival by administration of anti-CD45RB monoclonal antibody is due to alteration of CD45RBhi: CD45RBlo T-cell proportions. Am J Transplant 6(9):2023–2034
Lutz MB (2004) IL-3 in dendritic cell development and function: a comparison with GM-CSF and IL-4. Immunobiology 209(1–2):79–87
Malinowska M Wilkinson FL et al (2010) Genistein improves neuropathology and corrects behaviour in a mouse model of neurodegenerative metabolic disease. PLoS One 5(12): e14192.
Mellman I, Steinman RM (2001) Dendritic cells: specialized and regulated antigen processing machines. Cell 106(3):255–258
Moore D, Connock MJ et al (2008) The prevalence of and survival in Mucopolysaccharidosis I: hurler, hurler-scheie and scheie syndromes in the UK. Orphanet J Rare Dis 3:24
Morrissey PJ, Charrier K et al (1993) CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med 178(1):237–244
Muenzer J, Wraith JE et al (2009) Mucopolysaccharidosis I: management and treatment guidelines. Pediatrics 123(1):19–29
Ohmi K, Greenberg DS et al (2003) Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci U S A 100(4):1902–1907
Ohta T, Kinoshita T et al (1997) Requirement of the caspase-3/CPP32 protease cascade for apoptotic death following cytokine deprivation in hematopoietic cells. J Biol Chem 272(37):23111–23116
Peters PJ, Borst J et al (1991) Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med 173(5):1099–1109
Piccirillo CA, Shevach EM (2001) Cutting edge: control of CD8+ T cell activation by CD4+ CD25+ immunoregulatory cells. J Immunol 167(3):1137–1140
Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6(2):99–104
Powrie F, Carlino J et al (1996) A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med 183(6):2669–2674
Puri N, Roche PA (2008) Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc Natl Acad Sci U S A 105(7):2580–2585
Russell C, Hendson G et al (1998) Murine MPS I: insights into the pathogenesis of Hurler syndrome. Clin Genet 53(5):349–361
Sallusto F, Geginat J et al (2004) Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22:745–763
Settembre C, Fraldi A et al (2008) A block of autophagy in lysosomal storage disorders. Hum Mol Genet 17(1):119–129
Shen DT, Ma JS et al (2006) Activation of primary T lymphocytes results in lysosome development and polarized granule exocytosis in CD4+ and CD8+ subsets, whereas expression of lytic molecules confers cytotoxicity to CD8+ T cells. J Leukoc Biol 80(4):827–837
Shevach EM (2009) Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30(5):636–645
Swain SL, Bradley LM et al (1991) Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol Rev 123:115–144
Tang Q, Bluestone JA (2008) The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol 9(3):239–244
Ten Hove T, The Olle F et al (2004) Expression of CD45RB functionally distinguishes intestinal T lymphocytes in inflammatory bowel disease. J Leukoc Biol 75(6):1010–1015
Valenzuela J, Schmidt C et al (2002) The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol 169(12):6842–6849
Van Gool SW, Vermeiren J et al (1999) Blocking CD40 - CD154 and CD80/CD86 - CD28 interactions during primary allogeneic stimulation results in T cell anergy and high IL-10 production. Eur J Immunol 29(8):2367–2375
Wraith JE (1995) The mucopolysaccharidoses: a clinical review and guide to management. Arch Dis Child 72(3):263–267
Yoshimori T (2004) Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun 313(2):453–458
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Douglas A. Brooks
Sources of support: BWB and KL-S are supported by grants from the UK society for Mucopolysaccharide diseases. JEF and LDA are supported by grants from the New Start Charity and UHSM endowments.
Rights and permissions
About this article
Cite this article
Archer, L.D., Langford-Smith, K.J., Critchley, W.R. et al. Characterisation of the T cell and dendritic cell repertoire in a murine model of mucopolysaccharidosis I (MPS I). J Inherit Metab Dis 36, 257–262 (2013). https://doi.org/10.1007/s10545-012-9508-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-012-9508-8